Cell Metabolism ( IF 29.0 ) Pub Date : 2020-12-29 , DOI: 10.1016/j.cmet.2020.12.004 Anitta Kinga Sárvári 1 , Elvira Laila Van Hauwaert 1 , Lasse Kruse Markussen 1 , Ellen Gammelmark 1 , Ann-Britt Marcher 1 , Morten Frendø Ebbesen 2 , Ronni Nielsen 1 , Jonathan Richard Brewer 2 , Jesper Grud Skat Madsen 1 , Susanne Mandrup 1
|
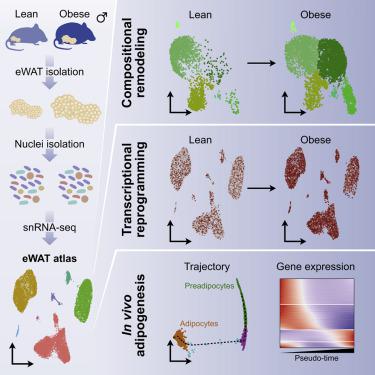
Adipose tissues display a remarkable ability to adapt to the dietary status. Here, we have applied single-nucleus RNA-seq to map the plasticity of mouse epididymal white adipose tissue at single-nucleus resolution in response to high-fat-diet-induced obesity. The single-nucleus approach allowed us to recover all major cell types and to reveal distinct transcriptional stages along the entire adipogenic trajectory from preadipocyte commitment to mature adipocytes. We demonstrate the existence of different adipocyte subpopulations and show that obesity leads to disappearance of the lipogenic subpopulation and increased abundance of the stressed lipid-scavenging subpopulation. Moreover, obesity is associated with major changes in the abundance and gene expression of other cell populations, including a dramatic increase in lipid-handling genes in macrophages at the expense of macrophage-specific genes. The data provide a powerful resource for future hypothesis-driven investigations of the mechanisms of adipocyte differentiation and adipose tissue plasticity.
中文翻译:

单核分辨率下附睾脂肪组织对饮食诱导肥胖的可塑性
脂肪组织显示出非凡的适应饮食状态的能力。在这里,我们应用单核 RNA-seq 以单核分辨率绘制了小鼠附睾白色脂肪组织的可塑性,以响应高脂肪饮食引起的肥胖。单核方法使我们能够恢复所有主要细胞类型,并沿着从前脂肪细胞定型到成熟脂肪细胞的整个脂肪形成轨迹揭示不同的转录阶段。我们证明了不同脂肪细胞亚群的存在,并表明肥胖导致脂肪生成亚群的消失和压力清除脂质亚群的丰度增加。此外,肥胖与其他细胞群的丰度和基因表达的重大变化有关,包括巨噬细胞中脂质处理基因的显着增加,但牺牲了巨噬细胞特异性基因。这些数据为未来脂肪细胞分化和脂肪组织可塑性机制的假设驱动研究提供了强大的资源。



























 京公网安备 11010802027423号
京公网安备 11010802027423号