Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2020-12-29 , DOI: 10.1016/j.abb.2020.108744 Alina A. Sofronova , Denis V. Pozdyshev , Kseniya V. Barinova , Vladimir I. Muronetz , Pavel I. Semenyuk
|
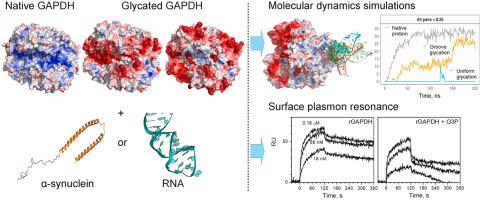
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) shows great diversity of functions, interaction partners and post-translational modifications. GAPDH undergoes glycation of positively charged residues in diabetic patient's tissues and therefore may change interaction with partners. The influence of GAPDH glycation on interaction with two important partners, α-synuclein and RNA, has been investigated in silico using molecular dynamics simulations and in vitro using surface plasmon resonance measurements. Since positively charged groove including substrate- and NAD+-binding sites is proposed as potential binding site for α-synuclein and RNA, GAPDH was glycated on residues in grooves and randomly distributed over the whole surface. Lysine residues were replaced with negatively charged carboxymethyl lysine as a widespread advanced glycation end product. As results, GAPDH glycation suppressed the interaction with α-synuclein and RNA. Although the modified GAPDH residues participated in binding with α-synuclein, no stable binding site with both glycated forms was observed. Glycation along the whole GAPDH surface completely suppressed interaction with RNA, whereas the alternative possible RNA binding site was identified in case of groove glycation. The findings were supported by direct measurement of the binding affinity. The obtained results clarify effect of glycation on GAPDH interaction with α-synuclein and RNA and elucidate a possible mechanism of interplay between glycation occurred in diabetes and neurodegenerative diseases, which GAPDH and α-synuclein are involved in.
中文翻译:

甘油醛-3-磷酸脱氢酶的糖基化抑制与α-突触核蛋白和RNA的结合
3-磷酸甘油醛脱氢酶(GAPDH)在功能,相互作用伴侣和翻译后修饰方面表现出极大的多样性。GAPDH在糖尿病患者的组织中经过带正电的残基糖基化,因此可能会改变与伴侣的相互作用。GAPDH糖基化对与两个重要伴侣α-突触核蛋白和RNA相互作用的影响已在计算机上使用分子动力学模拟进行了研究,并在体外使用了表面等离振子共振测量进行了研究。由于带正电荷的凹槽包括衬底和NAD +提出了结合位点作为α-突触核蛋白和RNA的潜在结合位点,GAPDH被糖化在凹槽中的残基上并随机分布在整个表面上。赖氨酸残基被带负电荷的羧甲基赖氨酸取代,作为一种广泛的高级糖化终产物。结果,GAPDH糖基化抑制了与α-突触核蛋白和RNA的相互作用。尽管修饰的GAPDH残基参与与α-突触核蛋白的结合,但未观察到两种糖化形式的稳定结合位点。沿整个GAPDH表面的糖基化作用完全抑制了与RNA的相互作用,而在凹槽糖基化的情况下,发现了其他可能的RNA结合位点。通过直接测量结合亲和力来支持该发现。











































 京公网安备 11010802027423号
京公网安备 11010802027423号