当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lithocholic Acid Derivatives as Potent Vitamin D Receptor Agonists
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-12-28 , DOI: 10.1021/acs.jmedchem.0c01420 Harue Sasaki 1 , Hiroyuki Masuno 2 , Haru Kawasaki 1 , Ayana Yoshihara 1 , Nobutaka Numoto 3 , Nobutoshi Ito 3 , Hiroaki Ishida 4 , Keiko Yamamoto 4 , Naoya Hirata 5 , Yasunari Kanda 5 , Emiko Kawachi 2 , Hiroyuki Kagechika 2 , Aya Tanatani 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2020-12-28 , DOI: 10.1021/acs.jmedchem.0c01420 Harue Sasaki 1 , Hiroyuki Masuno 2 , Haru Kawasaki 1 , Ayana Yoshihara 1 , Nobutaka Numoto 3 , Nobutoshi Ito 3 , Hiroaki Ishida 4 , Keiko Yamamoto 4 , Naoya Hirata 5 , Yasunari Kanda 5 , Emiko Kawachi 2 , Hiroyuki Kagechika 2 , Aya Tanatani 1
Affiliation
|
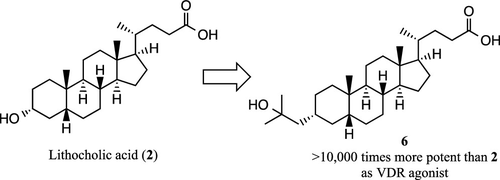
Lithocholic acid (2) was identified as a second endogenous ligand of vitamin D receptor (VDR), though its activity is very weak. In this study, we designed novel lithocholic acid derivatives based on the crystal structure of VDR–ligand-binding domain (LBD) bound to 2. Among the synthesized compounds, 6 bearing a 2-hydroxy-2-methylprop-1-yl group instead of the 3-hydroxy group at the 3α-position of 2 showed dramatically increased activity in HL-60 cell differentiation assay, being at least 10 000 times more potent than lithocholic acid (2) and 3 times more potent than 1α,25-dihydroxyvitamin D3 (1). Although the binding affinities of 6 and its epimer 7 were less than that of 1, their transactivation activities were greater than that of 1. X-ray structure analyses of VDR LBD bound to 6 or 7 showed that the binding positions of these compounds in the ligand-binding pocket are similar to that of 1.
中文翻译:

胆酸衍生物作为有效的维生素D受体激动剂
Lithocholic acid (2) was identified as a second endogenous ligand of vitamin D receptor (VDR), though its activity is very weak. In this study, we designed novel lithocholic acid derivatives based on the crystal structure of VDR–ligand-binding domain (LBD) bound to 2. Among the synthesized compounds, 6 bearing a 2-hydroxy-2-methylprop-1-yl group instead of the 3-hydroxy group at the 3α-position of 2 showed dramatically increased activity in HL-60 cell differentiation assay, being at least 10 000 times more potent than lithocholic acid (2) and 3 times more potent than 1α,25-dihydroxyvitamin D3 (1). Although the binding affinities of 6其差向异构体7小于1,其反式激活活性大于1。结合6或7的VDR LBD的X射线结构分析表明,这些化合物在配体结合袋中的结合位置与1相似。
更新日期:2021-01-14
中文翻译:

胆酸衍生物作为有效的维生素D受体激动剂
Lithocholic acid (2) was identified as a second endogenous ligand of vitamin D receptor (VDR), though its activity is very weak. In this study, we designed novel lithocholic acid derivatives based on the crystal structure of VDR–ligand-binding domain (LBD) bound to 2. Among the synthesized compounds, 6 bearing a 2-hydroxy-2-methylprop-1-yl group instead of the 3-hydroxy group at the 3α-position of 2 showed dramatically increased activity in HL-60 cell differentiation assay, being at least 10 000 times more potent than lithocholic acid (2) and 3 times more potent than 1α,25-dihydroxyvitamin D3 (1). Although the binding affinities of 6其差向异构体7小于1,其反式激活活性大于1。结合6或7的VDR LBD的X射线结构分析表明,这些化合物在配体结合袋中的结合位置与1相似。











































 京公网安备 11010802027423号
京公网安备 11010802027423号