当前位置:
X-MOL 学术
›
Bull. Korean Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 1,1‐Diisopropyl‐ or ‐Diphenyl ‐2,5‐dibromo‐ or ‐bis(trimethylsilyl)‐3,4‐diphenyl‐siloles and the Electrochemical Properties as Anode Materials for Lithium‐Ion Battery
Bulletin of the Korean Chemical Society ( IF 2.3 ) Pub Date : 2020-12-27 , DOI: 10.1002/bkcs.12193 Yoon‐ho Cho 1 , Young Min Jung 2 , Young Tae Park 1
Bulletin of the Korean Chemical Society ( IF 2.3 ) Pub Date : 2020-12-27 , DOI: 10.1002/bkcs.12193 Yoon‐ho Cho 1 , Young Min Jung 2 , Young Tae Park 1
Affiliation
|
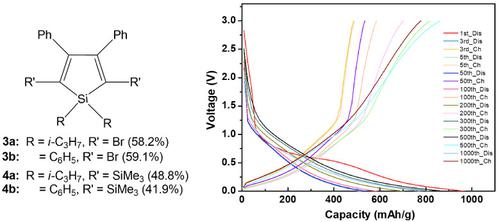
Intramolecular cyclization of 1,1‐diisopropyl‐ or diphenyl‐bis(phenylethynyl)‐silanes (2a and 2b) followed by bromination or trimethylsilylation were carried out to yield 1,1‐diisopropyl‐ or ‐diphenyl‐3,4‐diphenyl‐2,5‐dibromo‐siloles (3a and 3b) and 1,1‐diisoproyl‐ or ‐diphenyl‐3,4‐diphenyl‐2,5‐bis(trimethylsilyl)‐siloles (4a and 4b), respectively. The structures of 3a,b and 4a,b were confirmed using 1H, 13C, and 29Si NMR as well as FTIR spectroscopy. The absorption bands of the siloles 3a,b and 4a,b in THF were measured at 303–325 nm with the molar absorptivities of 1.85 × 103 ~ 2.18 × 103 cm−1·M−1. The excitation bands were measured at 347–376 nm and the emission peaks were measured at 409–445 nm. Cyclic voltammograms of 3a and 3b indicated oxidation peaks at 0.90 and 0.80 V and reduction peaks at −1.20 and −1.20 V, respectively. The cyclic voltammograms of 4a and 4b indicated two oxidation peaks between −0.05 and −0.95 V and two reduction peaks between −0.10 and −0.93 V, respectively. Compound 4a exhibits a better long cycle performance by almost 1000 cycles as compared that of 3a and 4b. The rate performance test of the anodes Li‐3a and Li‐4a exhibited better performance properties at various C rate than Li‐4b. According to discharge–charge curves, 4a shows one plateau at approximately 0.58 V of the first discharge curve and the initial discharge specific capacity of 972 mAh/g. The electrochemical impedance spectroscopy of 4a indicates low charge transfer resistance, good conductivity of the electrolyte, and fast chemical adsorption/desorption rate of electrolyte ions on electrode surface, due to the electronic structure of 4a.
中文翻译:

1,1-二异丙基或二苯基-2,5-二溴或双(三甲基甲硅烷基)-3,4-二苯基硅的合成及其作为锂离子电池负极材料的电化学性能
进行1,1-二异丙基或二苯基双(苯基乙炔基)硅烷的分子内环化反应(2a和2b),然后进行溴化或三甲基硅烷化反应,生成1,1-二异丙基或-二苯基-3,4-二苯基-2分别是,5-二溴硅烷(3a和3b)和1,1-二异丙基或二苯基-3,4-二苯基-2,5-双(三甲基甲硅烷基)-硅烷(4a和4b)。使用1 H,13 C和29 Si NMR以及FTIR光谱确认3a,b和4a,b的结构。筒仓3a,b的吸收带和图4A,4B在THF中在303-325 nm处测定为1.85×10的摩尔吸光系数3 〜2.18×10 3 厘米-1 ·男-1。在347-376 nm处测量了激发带,在409-445 nm处测量了发射峰。3a和3b的循环伏安图分别表示在0.90和0.80V的氧化峰和在-1.20和-1.20V的还原峰。4a和4b的循环伏安图分别表示在-0.05和-0.95 V之间的两个氧化峰和在-0.10和-0.93 V之间的两个还原峰。化合物4a与3a和4b相比,具有更好的长循环性能约1000个循环。阳极的速率性能测试锂3A和锂4a中在不同的C倍率比俪表现出更好的性能特性图4b。根据放电-充电曲线,4a在第一个放电曲线的约0.58 V处显示一个平稳段,初始放电比容量为972 mAh / g。4a的电化学阻抗谱表明,由于4a的电子结构,电荷转移电阻低,电解质的导电性好以及电解质离子在电极表面的化学吸附/解吸速率快。
更新日期:2020-12-27
中文翻译:

1,1-二异丙基或二苯基-2,5-二溴或双(三甲基甲硅烷基)-3,4-二苯基硅的合成及其作为锂离子电池负极材料的电化学性能
进行1,1-二异丙基或二苯基双(苯基乙炔基)硅烷的分子内环化反应(2a和2b),然后进行溴化或三甲基硅烷化反应,生成1,1-二异丙基或-二苯基-3,4-二苯基-2分别是,5-二溴硅烷(3a和3b)和1,1-二异丙基或二苯基-3,4-二苯基-2,5-双(三甲基甲硅烷基)-硅烷(4a和4b)。使用1 H,13 C和29 Si NMR以及FTIR光谱确认3a,b和4a,b的结构。筒仓3a,b的吸收带和图4A,4B在THF中在303-325 nm处测定为1.85×10的摩尔吸光系数3 〜2.18×10 3 厘米-1 ·男-1。在347-376 nm处测量了激发带,在409-445 nm处测量了发射峰。3a和3b的循环伏安图分别表示在0.90和0.80V的氧化峰和在-1.20和-1.20V的还原峰。4a和4b的循环伏安图分别表示在-0.05和-0.95 V之间的两个氧化峰和在-0.10和-0.93 V之间的两个还原峰。化合物4a与3a和4b相比,具有更好的长循环性能约1000个循环。阳极的速率性能测试锂3A和锂4a中在不同的C倍率比俪表现出更好的性能特性图4b。根据放电-充电曲线,4a在第一个放电曲线的约0.58 V处显示一个平稳段,初始放电比容量为972 mAh / g。4a的电化学阻抗谱表明,由于4a的电子结构,电荷转移电阻低,电解质的导电性好以及电解质离子在电极表面的化学吸附/解吸速率快。











































 京公网安备 11010802027423号
京公网安备 11010802027423号