当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microwave‐assisted synthesis of 2‐styrylquinoline‐4‐carboxylic acid derivatives to improve the toxic effect against Leishmania (Leishmania) amazonensis
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-12-27 , DOI: 10.1002/jhet.4217 Ayelen Luczywo 1 , Ismael Pretto Sauter 2 , Thalita Camêlo Silva Ferreira 2 , Mauro Cortez 2 , Gustavo P. Romanelli 3 , Gabriel Sathicq 3 , Silvia E. Asís 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-12-27 , DOI: 10.1002/jhet.4217 Ayelen Luczywo 1 , Ismael Pretto Sauter 2 , Thalita Camêlo Silva Ferreira 2 , Mauro Cortez 2 , Gustavo P. Romanelli 3 , Gabriel Sathicq 3 , Silvia E. Asís 1
Affiliation
|
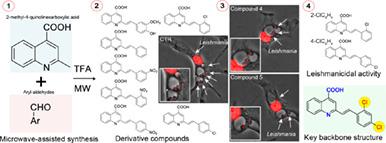
The identification of new compounds is urgent to develop safe and efficacious candidates for leishmaniasis treatment, especially from natural products as a potential source of active molecules against neglected tropical parasite diseases. Inspired by the efficacious quinoline alkaloid microbial effects, we have previously reported the synthesis and biological activity of 2‐phenylquinoline‐4‐carboxylic acids and poly‐substituted quinolines against parasites. In this work, a series of eighteen 2‐styryl‐4‐quinolinecarboxylic acids were synthesized under microwave irradiation settings obtaining from good to excellent yields (60%‐90%), shorter reaction times (2 minutes), and eco‐friendly experimental conditions. All these products were evaluated against infective forms of Leishmania (Leishmania) amazonensis, such as promastigotes and intracellular amastigotes, based on cytotoxicity assays, including host macrophage infection assays. Compounds 4 and 5 possessing a 2‐chloro or 4‐chlorostyryl moiety, respectively, were considered the most promising antileishmanial agents due to the parasite killing effect in intracellular forms inside infected macrophages. Thus, our results revealed that the 2‐styryl‐4‐quinolinecarboxylic acid backbone structure was essential for the activity against intracellular pathogens like L. (L.) amazonensis.
中文翻译:

微波辅助合成2-苯乙烯基喹啉-4-羧酸衍生物,以改善对亚马逊利什曼原虫(Leishmania)的毒性作用
鉴定新化合物迫在眉睫,以开发安全有效的利什曼病治疗候选药物,尤其是从天然产物中作为对抗被忽视的热带寄生虫疾病的活性分子的潜在来源。受有效的喹啉生物碱微生物作用的启发,我们先前已经报道了2-苯基喹啉-4-羧酸和多取代喹啉对寄生虫的合成和生物学活性。在这项工作中,在微波辐射设置下,合成了十八种2-苯乙烯基-4-喹啉羧酸,获得了良好至优异的收率(60%-90%),更短的反应时间(2分钟)和生态友好的实验条件。所有这些产品均针对亚马逊利什曼原虫(Leishmania)amazonensis的感染形式进行了评估(例如前鞭毛体和胞内变形虫),是基于细胞毒性分析(包括宿主巨噬细胞感染分析)的结果。化合物4和5分别具有2-氯或4-氯苯乙烯基部分,由于被感染的巨噬细胞以细胞内形式被寄生虫杀死,因此被认为是最有前途的抗疟原虫剂。因此,我们的结果表明2-苯乙烯基-4-喹啉羧酸主链结构对于抵抗诸如亚马逊L.(L.)的细胞内病原体的活性至关重要。
更新日期:2021-03-02
中文翻译:

微波辅助合成2-苯乙烯基喹啉-4-羧酸衍生物,以改善对亚马逊利什曼原虫(Leishmania)的毒性作用
鉴定新化合物迫在眉睫,以开发安全有效的利什曼病治疗候选药物,尤其是从天然产物中作为对抗被忽视的热带寄生虫疾病的活性分子的潜在来源。受有效的喹啉生物碱微生物作用的启发,我们先前已经报道了2-苯基喹啉-4-羧酸和多取代喹啉对寄生虫的合成和生物学活性。在这项工作中,在微波辐射设置下,合成了十八种2-苯乙烯基-4-喹啉羧酸,获得了良好至优异的收率(60%-90%),更短的反应时间(2分钟)和生态友好的实验条件。所有这些产品均针对亚马逊利什曼原虫(Leishmania)amazonensis的感染形式进行了评估(例如前鞭毛体和胞内变形虫),是基于细胞毒性分析(包括宿主巨噬细胞感染分析)的结果。化合物4和5分别具有2-氯或4-氯苯乙烯基部分,由于被感染的巨噬细胞以细胞内形式被寄生虫杀死,因此被认为是最有前途的抗疟原虫剂。因此,我们的结果表明2-苯乙烯基-4-喹啉羧酸主链结构对于抵抗诸如亚马逊L.(L.)的细胞内病原体的活性至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号