Microbial Pathogenesis ( IF 3.3 ) Pub Date : 2020-12-28 , DOI: 10.1016/j.micpath.2020.104705 Faruq Abdulla 1 , Zulkar Nain 2 , Md Moyazzem Hossain 3 , Shifath Bin Syed 2 , Md Shakil Ahmed Khan 2 , Utpal Kumar Adhikari 4
|
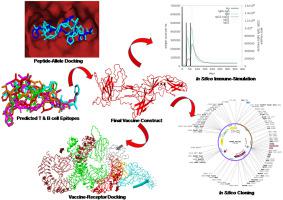
Hantaviruses are an emerging zoonotic group of rodent-borne viruses that are having serious implications on global public health due to the increase in outbreaks. Since there is no permanent cure, there is increasing interest in developing a vaccine against the hantavirus. This research aimed to design a robust cross-protective subunit vaccine using a novel immunoinformatics approach. After careful evaluation, the best predicted cytotoxic & helper T-cell and B-cell epitopes from nucleocapsid proteins, glycoproteins, RdRp proteins, and non-structural proteins were considered as potential vaccine candidates. Among the four generated vaccine models with different adjuvant, the model with toll-like receptor-4 (TLR-4) agonist adjuvant was selected because of its high antigenicity, non-allergenicity, and structural quality. The selected model was 654 amino acids long and had a molecular weight of 70.5 kDa, which characterizes the construct as a good antigenic vaccine candidate. The prediction of the conformational B-lymphocyte (CBL) epitope secured its ability to induce the humoral response. Thereafter, disulfide engineering improved vaccine stability. Afterwards, the molecular docking confirmed a good binding affinity of −1292 kj/mol with considered immune receptor TLR-4 and the dynamics simulation showed high stability of the vaccine-receptor complex. Later, the in silico cloning confirmed the better expression of the constructed vaccine protein in E. coli K12. Finally, in in silico immune simulation, significantly high levels of immunoglobulin M (IgM), immunoglobulin G1 (IgG1), cytotoxic & helper T lymphocyte (CTL & HTL) populations, and numerous cytokines such as interferon-γ (IFN-γ), interleukin-2 (IL-2) etc. were found as coherence with actual immune response and also showed faster antigen clearance for repeated exposures. Nonetheless, experimental validation can demonstrate the safety and cross-protective ability of the proposed vaccine to fight against hantavirus infection.
中文翻译:

汉坦病毒全蛋白质组的全面筛选和设计用于汉坦病毒交叉保护的多表位亚单位疫苗:结构疫苗学和免疫信息学研究
汉坦病毒是一种新兴的由啮齿动物传播的人畜共患病毒,由于疫情的增加,它们对全球公共卫生产生了严重影响。由于没有永久的治愈方法,人们对开发针对汉坦病毒的疫苗越来越感兴趣。本研究旨在使用一种新的免疫信息学方法设计一种强大的交叉保护亚单位疫苗。经过仔细评估,来自核衣壳蛋白、糖蛋白、RdRp 蛋白和非结构蛋白的最佳预测细胞毒性和辅助 T 细胞和 B 细胞表位被认为是潜在的候选疫苗。在生成的四种不同佐剂的疫苗模型中,选择了Toll样受体4(TLR-4)激动剂佐剂的模型,因为它具有高抗原性、非过敏性和结构质量。所选模型长 654 个氨基酸,分子量为 70.5 kDa,这表明该构建体是一种良好的抗原性疫苗候选物。构象 B 淋巴细胞 (CBL) 表位的预测确保了其诱导体液反应的能力。此后,二硫化物工程提高了疫苗的稳定性。之后,分子对接证实了与所考虑的免疫受体 TLR-4 具有 -1292 kj/mol 的良好结合亲和力,动力学模拟显示疫苗-受体复合物的高稳定性。后来,之后,分子对接证实了与所考虑的免疫受体 TLR-4 具有 -1292 kj/mol 的良好结合亲和力,动力学模拟显示疫苗-受体复合物的高稳定性。后来,之后,分子对接证实了与所考虑的免疫受体 TLR-4 具有 -1292 kj/mol 的良好结合亲和力,动力学模拟显示疫苗-受体复合物的高稳定性。后来,计算机克隆证实了构建的疫苗蛋白在大肠杆菌K12中的更好表达。最后,在计算机免疫模拟中,显着高水平的免疫球蛋白 M (IgM)、免疫球蛋白 G1 (IgG1)、细胞毒性和辅助 T 淋巴细胞 (CTL & HTL) 群体,以及众多细胞因子,如干扰素-γ (IFN-γ),发现白细胞介素 2 (IL-2) 等与实际免疫反应具有一致性,并且对于重复暴露也显示出更快的抗原清除速度。尽管如此,实验验证可以证明所提出的疫苗对抗汉坦病毒感染的安全性和交叉保护能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号