Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-12-28 , DOI: 10.1016/j.bmc.2020.115969 Eslam M H Ali 1 , Mohammed S Abdel-Maksoud 2 , Rasha Mohamed Hassan 2 , Karim I Mersal 3 , Usama M Ammar 4 , Choi Se-In 5 , Han He-Soo 5 , Hee-Kwon Kim 6 , Anna Lee 7 , Kyung-Tae Lee 5 , Chang-Hyun Oh 3
|
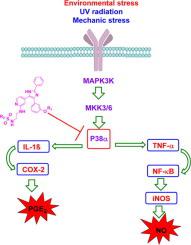
P38α/MAPK14 is intracellular signalling regulator involved in biosynthesis of inflammatory mediator cytokines (TNF-α, IL-1, IL-6, and IL-1b), which induce the production of inflammatory proteins (iNOS, NF-kB, and COX-2). In this study, drug repurposing strategies were followed to repositioning of a series of B-RAF V600E imidazol-5-yl pyridine inhibitors to inhibit P38α kinase. A group 25 reported P38α kinase inhibitors were used to build a pharmacophore model for mapping the target compounds and proving their affinity for binding in P38α active site. Target compounds were evaluated for their potency against P38α kinase, compounds 11a and 11d were the most potent inhibitors (IC50 = 47 nM and 45 nM, respectively).
In addition, compound 11d effectively inhibited the production of proinflammatory cytokines TNF-α, 1L-6, and 1L-1β in LPS-induced RAW 264.7 macrophages with IC50 values of 78.03 nM, 17.6 µM and 82.15 nM, respectively.
The target compounds were tested for their anti-inflammatory activity by detecting the reduction of Nitric oxide (NO) and prostaglandin (PGE2) production in LPS-stimulated RAW 264.7 macrophages. Compound 11d exhibited satisfied inhibitory activity of the production of PGE2 and NO with IC50 values of 0.29 µM and 0.61 µM, respectively. Molecular dynamics simulations of the most potent inhibitor 11d were carried out to illustrate its conformational stability in the binding site of P38α kinase.
中文翻译:

作为 p38α/MAPK14 抑制剂的咪唑-5-基吡啶衍生物的设计、合成和抗炎活性
P38α/MAPK14 是细胞内信号调节剂,参与炎症介质细胞因子(TNF-α、IL-1、IL-6 和 IL-1b)的生物合成,诱导炎症蛋白(iNOS、NF-kB 和 COX- 2)。在这项研究中,遵循药物再利用策略重新定位一系列 B-RAF V600E咪唑-5-基吡啶抑制剂以抑制 P38α 激酶。一组 25 报告的 P38α 激酶抑制剂用于构建药效团模型,用于绘制目标化合物并证明它们与 P38α 活性位点的结合亲和力。评估了目标化合物对 P38α 激酶的效力,化合物11a和11d是最有效的抑制剂(IC 50 分别 为 47 nM 和 45 nM)。
此外,化合物11d 可有效抑制 LPS 诱导的 RAW 264.7 巨噬细胞中促炎细胞因子 TNF-α、1L-6 和 1L-1β 的产生,IC 50值分别为 78.03 nM、17.6 µM 和 82.15 nM。
通过检测 LPS 刺激的 RAW 264.7 巨噬细胞中一氧化氮 (NO) 和前列腺素 (PGE2) 产生的减少,测试了目标化合物的抗炎活性。化合物11d表现出令人满意的PGE2和NO生成抑制活性,IC 50值分别为0.29 μM和0.61 μM。进行了最有效抑制剂11d 的分子动力学模拟,以说明其在 P38α 激酶结合位点的构象稳定性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号