Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2020-12-26 , DOI: 10.1016/j.jcou.2020.101408 Georgios Varvoutis , Maria Lykaki , Eleni Papista , Sόnia A.C. Carabineiro , Antonios C. Psarras , Georgios E. Marnellos , Michalis Konsolakis
|
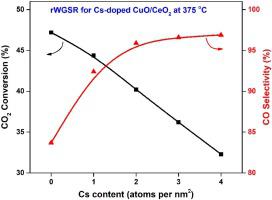
The reaction of captured carbon dioxide with renewable hydrogen towards the eventual indirect production of liquid hydrocarbons via CO2 reduction to CO (reverse water-gas shift reaction, rWGS) is a promising pathway in the general scheme of worldwide CO2 valorization. Copper-ceria oxides have been largely employed as rWGS catalysts owing to their unique properties linked to copper-ceria interactions. Here, we report on the fine-tuning of CuO/CeO2 composites by means of alkali promotion. In particular, this work aims at exploring the effect of cesium doping (0–4 atoms Cs per nm2) on co-precipitated CuO/CeO2 catalysts under CO2 hydrogenation conditions. The as-prepared samples were characterized by N2 physisorption, X-ray diffraction (XRD), H2-temperature programmed reduction (H2-TPR), X-ray photoelectron spectroscopy (XPS), CO2-temperature programmed desorption (CO2-TPD), Fourier-transform infrared spectroscopy (FTIR) of pyridine adsorption and CO-diffuse reflectance Fourier-transform infrared spectroscopy (CO-DRIFTS). The results demonstrated that a low amount of Cs exerted a beneficial effect on CO selectivity, inhibiting, however, CO2 conversion. Specifically, a doping of 2 atoms Cs per nm2 offers > 96 % CO selectivity and equilibrium CO2 conversion at temperatures as low as 430 °C, whereas further increase in cesium loading had no additional impact. The present findings can be mainly interpreted on a basis of the alkali effect on the textural and acid/base properties; Cs doping results in a significant reduction of the surface area and thus to a lower population of active sites for CO2 conversion, whereas it enhances the formation of basic sites and the stabilization of partially reduced Cu+ species, favoring CO selectivity.
中文翻译:

碱掺杂对CuO / CeO 2催化剂表面化学性质和CO 2加氢性能的影响
捕获的二氧化碳与可再生氢的反应,通过将CO 2还原为CO最终间接产生液态烃(逆水煤气变换反应,rWGS),是在全球范围内进行CO 2增值的一般方案中的一个有希望的途径。氧化铜-氧化铈由于其与氧化铜-氧化铈相互作用相关的独特性能而被广泛用作rWGS催化剂。在这里,我们报告了通过碱促进对CuO / CeO 2复合材料的微调。特别是,这项工作旨在探索铯掺杂(0-4个原子Cs / nm 2)对CO 2下共沉淀CuO / CeO 2催化剂的影响。氢化条件。制备的样品的特征在于N 2物理吸附,X射线衍射(XRD),H 2程序升温还原(H 2 -TPR ),X射线光电子能谱(XPS),CO 2程序升温解吸(CO)2 -TPD),吡啶吸附的傅里叶变换红外光谱法(FTIR)和CO扩散反射率傅里叶变换红外光谱法(CO-DRIFTS)。结果表明,少量的Cs对CO的选择性发挥了有益的作用,但是抑制了CO 2的转化。具体而言,每nm 2掺杂2个原子Cs可提供> 96%的CO选择性和平衡的CO 2。在低至430°C的温度下转化,而铯负载的进一步增加没有其他影响。本发现主要可以根据碱对质地和酸/碱性质的影响来解释。Cs掺杂会导致表面积显着减少,从而减少用于CO 2转化的活性位点的数量,而它会增强碱性位点的形成和部分还原的Cu +物种的稳定性,有利于CO的选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号