Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.1 ) Pub Date : 2020-12-26 , DOI: 10.1016/j.jphotochem.2020.113110 Judit Michnyóczki , Virág Kiss , Katalin Ősz
|
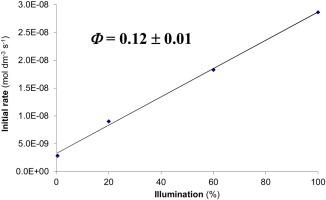
The photooxidation of water by aqueous cerium(IV) has been investigated in dilute sulfuric acid quantitatively using a diode array spectrophotometer. An overview of experimental data on the speciation of cerium(IV) showed that several different absorbing species are present under almost all conditions, which makes straightforward interpretation of photochemical data very difficult. At submillimolar levels of cerium(IV) in 0.10 mol dm–3 sulfuric acid, a consistent quantum yield of 0.12 for the loss of cerium(IV) could be determined, but even under these conditions, clear evidence of a parallel non-photochemical pathway was obtained. At higher concentrations of the metal ion, significant deviations from the expected direct proportionality between the illumination ratio and the initial rate were confirmed, which can only be interpreted by a complicated system of parallel, coupled photochemical and non-photochemical pathways. The inhibitory effect of aqueous cerium(III) in the process was also demonstrated. Based on indirect evidence, it seems likely that sulfate ion radicals play a significant role in the mechanism.
中文翻译:

用二极管阵列分光光度计动力学研究硫酸铈水溶液对水的光氧化反应
已使用二极管阵列分光光度计在稀硫酸中定量研究了铈(IV)水溶液对水的光氧化作用。铈(IV)形态的实验数据概述表明,在几乎所有条件下都存在几种不同的吸收物种,这使得对光化学数据的直接解释非常困难。铈(IV)的亚毫摩尔水平为0.10 mol dm –3可以测定硫酸在铈(IV)损失方面的始终如一的量子产率,为0.12,但是即使在这些条件下,也获得了平行的非光化学途径的明确证据。在较高的金属离子浓度下,证实了与照明比率和初始速率之间的预期直接比例有显着偏差,这只能由平行,耦合的光化学和非光化学途径的复杂系统来解释。还证明了铈(III)水溶液在该过程中的抑制作用。根据间接证据,似乎硫酸根离子在该机理中起着重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号