当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of new pyrazoles, oxadiazoles, triazoles, pyrrolotriazines, and pyrrolotriazepines as potential cytotoxic agents
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-12-24 , DOI: 10.1002/jhet.4216 Ameen Ali Abu‐Hashem 1, 2
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-12-24 , DOI: 10.1002/jhet.4216 Ameen Ali Abu‐Hashem 1, 2
Affiliation
|
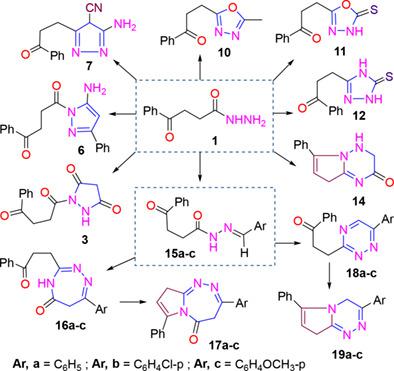
4‐Oxo‐4‐phenylbutanehydrazide (1) reacted with many active methylene reagents such as acetylacetone, diethylmalonate, ethylacetoacetate, ethylcyanoacetate, benzoyl‐acetonitrile, and malononitrile under neat conditions to afford the corresponding pyrazoles (2–7), also, treatment of butanehydrazide (1) with electrophilic reagents as triethylorthoformate, dimethylformamide‐dimethylacetal, acetic anhydride, and carbon disulfide to give 1,3,4‐oxadiazoles (8,10,11) and N′‐acetyl‐butanehydrazide (9). Reacted of butanehydrazide (1) with potassium thiocyanate gave 1,2,4‐triazoles (12). Similarly, treatment of (1) with chloroacetamide gave 1,2,4‐triazinones (13). The pyrrolotriazinones (14) was obtained by cyclization of (13). Also, butanehydrazide (1) was utilized as a starting material for the synthesized of new Schiff bases as N′‐(4‐sub‐benzylidene)‐phenylbutane‐hydrazide (15a‐c), which are used as an initiative to prepare new compounds such as 1,2,4‐triazepinones (16a‐c), pyrrolotriazepinones (17a‐c), 1,2,4‐triazines (18a‐c), and pyrrolotriazines (19a‐c) by reacted of (15a‐c) with each chloroacetamide or formamide. The chemical structure of the newly prepared compounds was determined through the spectrum data, including IR, NMR, and MS. The prepared compounds were tested for their in vitro antitumor activities. The compounds 17a‐c, 16a‐c, and 19a‐c displayed activity against several types of cancer cell lines.
中文翻译:

合成新的吡唑,恶二唑,三唑,吡咯并三嗪和吡咯并ze庚因作为潜在的细胞毒剂
4-氧代-4- phenylbutanehydrazide (1)具有许多活性亚甲基的试剂如乙酰丙酮,丙二酸二乙酯,乙酰乙酸乙酯,氰基乙酸乙酯,苯甲酰基乙腈,丙二腈和整齐的条件,得到相应的吡唑类下进行反应(2-7) ,另外,治疗的丁酰肼(1)与亲电试剂如原甲酸三乙酯,二甲基甲酰胺-二甲基乙缩醛,乙酸酐和二硫化碳,得到1,3,4-恶二唑(8,10,11)和N'-乙酰基-丁酰肼(9)。丁酰肼(1)与硫氰酸钾反应生成1,2,4-三唑(12)。同样,对(1)的处理用氯乙酰胺制得1,2,4-三嗪酮(13)。吡咯并三嗪酮(14)通过(13)的环化获得。此外,丁酰肼(1)被用作合成新席夫碱(N '-(4-亚苄基)-苯基丁烷酰肼(15a-c))的起始原料,用于制备新化合物如1,2,4-三氮杂吡啶酮(16a-c),吡咯并三氮杂吡啶酮(17a-c),1,2,4-三嗪(18a-c)和吡咯并三嗪(19a-c)通过(15a-c)反应与每种氯乙酰胺或甲酰胺一起使用。通过光谱数据(包括IR,NMR和MS)确定了新制备化合物的化学结构。测试了所制备的化合物的体外抗肿瘤活性。化合物17a-c,16a-c和19a-c对多种类型的癌细胞系均具有活性。
更新日期:2021-03-02
中文翻译:

合成新的吡唑,恶二唑,三唑,吡咯并三嗪和吡咯并ze庚因作为潜在的细胞毒剂
4-氧代-4- phenylbutanehydrazide (1)具有许多活性亚甲基的试剂如乙酰丙酮,丙二酸二乙酯,乙酰乙酸乙酯,氰基乙酸乙酯,苯甲酰基乙腈,丙二腈和整齐的条件,得到相应的吡唑类下进行反应(2-7) ,另外,治疗的丁酰肼(1)与亲电试剂如原甲酸三乙酯,二甲基甲酰胺-二甲基乙缩醛,乙酸酐和二硫化碳,得到1,3,4-恶二唑(8,10,11)和N'-乙酰基-丁酰肼(9)。丁酰肼(1)与硫氰酸钾反应生成1,2,4-三唑(12)。同样,对(1)的处理用氯乙酰胺制得1,2,4-三嗪酮(13)。吡咯并三嗪酮(14)通过(13)的环化获得。此外,丁酰肼(1)被用作合成新席夫碱(N '-(4-亚苄基)-苯基丁烷酰肼(15a-c))的起始原料,用于制备新化合物如1,2,4-三氮杂吡啶酮(16a-c),吡咯并三氮杂吡啶酮(17a-c),1,2,4-三嗪(18a-c)和吡咯并三嗪(19a-c)通过(15a-c)反应与每种氯乙酰胺或甲酰胺一起使用。通过光谱数据(包括IR,NMR和MS)确定了新制备化合物的化学结构。测试了所制备的化合物的体外抗肿瘤活性。化合物17a-c,16a-c和19a-c对多种类型的癌细胞系均具有活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号