当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
γ‐d‐Glutamyl‐meso‐diaminopimelic acid induces autophagy in bovine hepatocytes during nucleotide‐binding oligomerization domain 1‐mediated inflammation
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-12-24 , DOI: 10.1002/jcp.30227 Animesh Chandra Roy 1, 2 , Guangjun Chang 1 , Shipra Roy 1 , Nana Ma 1 , Qianyun Gao 1 , Xiangzhen Shen 1
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-12-24 , DOI: 10.1002/jcp.30227 Animesh Chandra Roy 1, 2 , Guangjun Chang 1 , Shipra Roy 1 , Nana Ma 1 , Qianyun Gao 1 , Xiangzhen Shen 1
Affiliation
|
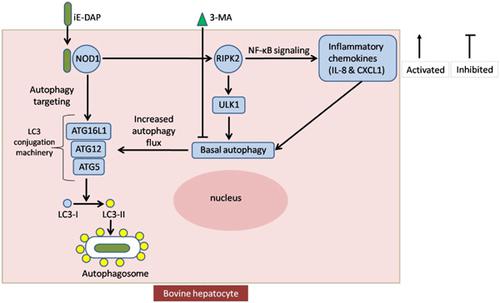
Autophagy is a crucial cellular homeostatic process and an important part of the host defense system. Dysfunction in autophagy enhances tissue susceptibility to infection and multiple diseases. However, the role of nucleotide oligomerization domain 1 (NOD1) in autophagy in bovine hepatocytes is not well known. Therefore, our aim was to study the contribution of NOD1 to autophagy during inflammation in response to a specific ligand γ‐d‐glutamyl‐meso‐diaminopimelic acid (iE‐DAP). To achieve this aim, hepatocytes separated from cows at ∼160 days in milk (DIM) were divided into six groups: the nontreated control (CON) group, the rapamycin‐treated (RAP) group as a positive control, the iE‐DAP‐treated (DAP) group, the 3‐MA‐treated (MA) group, the rapamycin with 3‐MA (RM) group, and the iE‐DAP with 3‐MA (DM) group. iE‐DAP administration significantly increased the mRNA expression of NOD1, ATG16L1, RIPK2, ULK1, AMBRA1, DFCP1, WIPI1, ATG5, ATG7, ATG10, ATG4A, IκBα, NF‐κB, CXCL1, IL‐8, and STAT6 and significantly decreased PIK3C3. The protein expression of NOD1, p‐IκBα, p‐NF‐κB/p‐p65, LC3‐II, ATG5, and beclin 1 were significantly upregulated and that of SQSTM1/p62, p‐mTOR, and FOXA2 were significantly downregulated in response to iE‐DAP. iE‐DAP also induced the formation of LC3‐GFP autophagic puncta in bovine hepatocytes. We also knocked down the NOD1 with siRNA. NOD1 silencing suppressed the autophagy and inflammation‐related genes and proteins. The application of the autophagy inhibitor increased the expression of inflammatory molecules and alleviated autophagy‐associated molecules. Taken together, these findings suggest that NOD1 is a key player for regulating both ATG16L1 and RIPK2‐ULK1 directed autophagy during inflammation in response to iE‐DAP in bovine hepatocytes.
中文翻译:

γ-d-谷氨酰-内消旋二氨基庚二酸在核苷酸结合寡聚化结构域1介导的炎症过程中诱导牛肝细胞自噬
自噬是一个关键的细胞稳态过程,也是宿主防御系统的重要组成部分。自噬功能障碍增强了组织对感染和多种疾病的易感性。然而,核苷酸寡聚化结构域 1 (NOD1) 在牛肝细胞自噬中的作用尚不清楚。因此,我们的目的是研究 NOD1 在炎症期间响应特定配体 γ- d-谷氨酰-内消旋体对自噬的贡献。-二氨基庚二酸(iE-DAP)。为了实现这一目标,在牛奶(DIM)中从大约 160 天的奶牛中分离出的肝细胞分为六组:未处理的对照组(CON)、雷帕霉素处理的(RAP)组作为阳性对照、iE-DAP-治疗(DAP)组、3-MA 治疗(MA)组、雷帕霉素加 3-MA(RM)组和 iE-DAP 加 3-MA(DM)组。iE-DAP 给药显着增加 NOD1、ATG16L1、RIPK2、ULK1、AMBRA1、DFCP1、WIPI1、ATG5、ATG7、ATG10、ATG4A、IκBα、NF-κB、CXCL1、IL-8 和 STAT3C3 的 mRNA 表达并显着降低 PIK3C3 . NOD1、p-IκBα、p-NF-κB/p-p65、LC3-II、ATG5 和 beclin 1 的蛋白表达显着上调,而 SQSTM1/p62、p-mTOR 和 FOXA2 的蛋白表达显着下调到 iE-DAP。iE-DAP 还诱导牛肝细胞中 LC3-GFP 自噬点的形成。我们还用 siRNA 击倒了 NOD1。NOD1 沉默抑制了自噬和炎症相关基因和蛋白质。自噬抑制剂的应用增加了炎症分子的表达并减轻了自噬相关分子。综上所述,这些发现表明 NOD1 是调节 ATG16L1 和 RIPK2-ULK1 在炎症过程中对牛肝细胞中 iE-DAP 的定向自噬的关键参与者。
更新日期:2020-12-24
中文翻译:

γ-d-谷氨酰-内消旋二氨基庚二酸在核苷酸结合寡聚化结构域1介导的炎症过程中诱导牛肝细胞自噬
自噬是一个关键的细胞稳态过程,也是宿主防御系统的重要组成部分。自噬功能障碍增强了组织对感染和多种疾病的易感性。然而,核苷酸寡聚化结构域 1 (NOD1) 在牛肝细胞自噬中的作用尚不清楚。因此,我们的目的是研究 NOD1 在炎症期间响应特定配体 γ- d-谷氨酰-内消旋体对自噬的贡献。-二氨基庚二酸(iE-DAP)。为了实现这一目标,在牛奶(DIM)中从大约 160 天的奶牛中分离出的肝细胞分为六组:未处理的对照组(CON)、雷帕霉素处理的(RAP)组作为阳性对照、iE-DAP-治疗(DAP)组、3-MA 治疗(MA)组、雷帕霉素加 3-MA(RM)组和 iE-DAP 加 3-MA(DM)组。iE-DAP 给药显着增加 NOD1、ATG16L1、RIPK2、ULK1、AMBRA1、DFCP1、WIPI1、ATG5、ATG7、ATG10、ATG4A、IκBα、NF-κB、CXCL1、IL-8 和 STAT3C3 的 mRNA 表达并显着降低 PIK3C3 . NOD1、p-IκBα、p-NF-κB/p-p65、LC3-II、ATG5 和 beclin 1 的蛋白表达显着上调,而 SQSTM1/p62、p-mTOR 和 FOXA2 的蛋白表达显着下调到 iE-DAP。iE-DAP 还诱导牛肝细胞中 LC3-GFP 自噬点的形成。我们还用 siRNA 击倒了 NOD1。NOD1 沉默抑制了自噬和炎症相关基因和蛋白质。自噬抑制剂的应用增加了炎症分子的表达并减轻了自噬相关分子。综上所述,这些发现表明 NOD1 是调节 ATG16L1 和 RIPK2-ULK1 在炎症过程中对牛肝细胞中 iE-DAP 的定向自噬的关键参与者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号