Catalysis Today ( IF 5.2 ) Pub Date : 2020-12-25 , DOI: 10.1016/j.cattod.2020.12.004 Andrey Zagoruiko , Pavel Mikenin
|
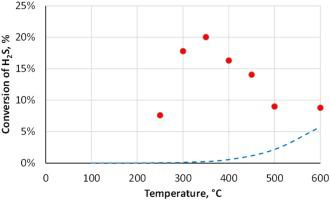
The research was devoted to experimental study of low-temperature cyclic chemisorption-catalytic process for H2S decomposition, including the identification of optimal chemisorbent-catalysts and quantitative investigation of process characteristics. The study involved sulfides of transient metals (Fe, Co and Ni) both in bulk and supported forms. The highest efficiency was achieved with bulk sulfides NiS, CoS and especially FeS. Sorption capacity of up to 10 % by weight is observed for these systems, while all the hydrogen sulfide is absorbed irreversibly. It was shown that at elevated temperatures (250−600 °C) the reaction can proceed in a quasi-stationary mode, in which the conversion of hydrogen sulfide and the yield of hydrogen are observed almost unchanged during rather long period of time. In this case, the conversion of H2S and the yield of H2 significantly exceed the equilibrium values for direct H2S decomposition reaction, thus indicating a noticeable contribution of chemisorption processes. In the optimal temperature range (350−400 °C), the hydrogen yield on these systems varies from 11 to 95 % with an average value of 26–34 % per cycle.
中文翻译:

在循环化学吸附催化过程中将硫化氢分解成元素
该研究致力于H 2的低温循环化学吸附催化过程的实验研究。S的分解,包括最佳化学吸附剂催化剂的鉴定和过程特征的定量研究。该研究涉及散装和支撑形式的过渡金属(Fe,Co和Ni)的硫化物。使用块状硫化物NiS,CoS尤其是FeS可获得最高的效率。这些系统的吸附能力高达10%(重量),而所有的硫化氢都被不可逆地吸收。结果表明,在升高的温度(250-600°C)下,反应可以以准平稳模式进行,其中在相当长的一段时间内观察到硫化氢的转化率和氢的收率几乎没有变化。在这种情况下,H的转化2 S和H的产量2明显超过直接H 2 S分解反应的平衡值,因此表明化学吸附过程的显着贡献。在最佳温度范围(350-400°C)中,这些系统的氢气产率在11%至95%之间变化,每个循环的平均值为26-34%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号