Acta Biomaterialia ( IF 9.7 ) Pub Date : 2020-12-25 , DOI: 10.1016/j.actbio.2020.12.045 Ya Xiao , Mengran Xu , Na Lv , Chen Cheng , Pei Huang , Jiabin Li , Yi Hu , Ming Sun
|
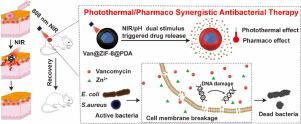
The serious threat of drug-resistant bacterial pathogens has arisen through overuse of antibiotics. Photothermal therapy (PTT) has come to prominence as viable alternative strategy for antibacterial therapy. In this work, we report a NIR/pH dual stimuli-responsive antibacterial formulation based on zeolitic imidazolate frameworks-8 (ZIF-8) with strong antibacterial activity that combines photothermal heating with enhanced antibiotic delivery. ZIF-8 with polydopamine (PDA) surface modification was used to encapsulate the antibiotic vancomycin to construct a dual stimuli-responsive antimicrobial formulation (Van@ZIF-8@PDA). This treatment was tested against Gram-positive Mu50 (a vancomycin-intermediate S. aureus reference strain). Results showed that the PDA coating improved ZIF-8 stability and dispersion, while also conferring a high photothermal conversion efficiency. Hyperthermia activated by near-infrared (NIR) light irradiation, in conjunction with pH-dependent nanoparticle degradation to release vancomycin, enabled tight control of drug delivery that functioned synergistically in the elimination of both planktonic bacteria prior to biofilm formation and established biofilms. We found that this combined formulation compromises cell structure while also degrading bacterial DNA. Moreover, further investigation showed that the Van@ZIF-8@PDA nanoparticles exhibit good biocompatibility, with low toxicity toward host organs and tissues, while also reducing the antibiotic concentration needed for effective bacterial control. Finally, we treated Mu50 in a mouse model of skin abscess and found that Van@ZIF-8@PDA was effective and safe in vivo. Cumulatively, this study shows that this NIR/pH dual stimuli-responsive nanoparticle-based formulation offers a promising potential strategy for clinical application against bacterial infection that circumvents antibiotic resistance.
中文翻译:

基于双重刺激响应金属-有机骨架的纳米系统,用于协同光热/药物抗菌治疗
过度使用抗生素已引起耐药细菌病原体的严重威胁。光热疗法(PTT)已成为抗菌疗法的可行替代策略。在这项工作中,我们报告了一种基于沸石咪唑盐骨架8(ZIF-8)的NIR / pH双重刺激响应抗菌制剂,该制剂具有强大的抗菌活性,结合了光热加热和增强的抗生素递送能力。使用具有聚多巴胺(PDA)表面修饰的ZIF-8来封装抗生素万古霉素,以构建双重刺激响应的抗菌制剂(Van @ ZIF-8 @ PDA)。该治疗针对革兰氏阳性Mu50(万古霉素中间体金黄色葡萄球菌)进行了测试参考菌株)。结果表明,PDA涂层改善了ZIF-8的稳定性和分散性,同时还赋予了较高的光热转换效率。通过近红外(NIR)光照射激活的热疗,再加上pH依赖的纳米颗粒降解以释放万古霉素,可实现对药物递送的严格控制,从而在消除生物膜形成之前的浮游细菌和建立生物膜方面具有协同作用。我们发现该组合制剂损害细胞结构,同时还降解细菌DNA。此外,进一步的研究表明,Van @ ZIF-8 @ PDA纳米颗粒具有良好的生物相容性,对宿主器官和组织的毒性低,同时还降低了有效控制细菌所需的抗生素浓度。最后,体内。累积地,这项研究表明,这种基于NIR / pH双刺激响应纳米颗粒的制剂为临床应用提供了一种有前途的潜在策略,可用于抵抗细菌耐药性的细菌感染。



























 京公网安备 11010802027423号
京公网安备 11010802027423号