当前位置:
X-MOL 学术
›
Anal. Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A simple, rapid and low-cost spectrophotometric method for irinotecan quantification in human plasma and in pharmaceutical dosage forms
Analytical Methods ( IF 2.7 ) Pub Date : 2020-12-8 , DOI: 10.1039/d0ay02201b Georgia Eleni Tsotsou 1, 2, 3, 4, 5 , Panagiota Gkotzamani 1, 2, 3, 4, 5 , Victoria Petro 1, 2, 3, 4, 5 , Ariadne Argyropoulou 1, 2, 3, 4, 5 , Petros Karkalousos 1, 2, 3, 4, 5
Analytical Methods ( IF 2.7 ) Pub Date : 2020-12-8 , DOI: 10.1039/d0ay02201b Georgia Eleni Tsotsou 1, 2, 3, 4, 5 , Panagiota Gkotzamani 1, 2, 3, 4, 5 , Victoria Petro 1, 2, 3, 4, 5 , Ariadne Argyropoulou 1, 2, 3, 4, 5 , Petros Karkalousos 1, 2, 3, 4, 5
Affiliation
|
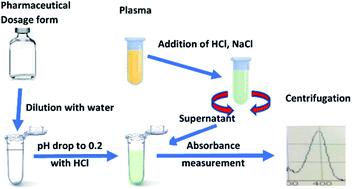
Irinotecan is an anticancer drug for which significant benefits from personalised dosing are expected. Quick procedures are therefore essential for monitoring irinotecan in treated patients. The objective of this work was to develop and validate a rapid and simple visible spectrophotometric method for quantitative determination of irinotecan in pharmaceutical dosage forms and to further investigate its usefulness for irinotecan analysis in plasma. Based on the shift of the irinotecan 355/368 nm-peak at very low pH (0.2) to 400 nm, we established a linear relationship between absorbance at 400 nm and irinotecan concentration in dilutions of an irinotecan solution for injection (R2 ≥ 0.999) and in plasma containing irinotecan (R2 ≥ 0.995). Background absorbance correction at 455 nm was essential to minimise background interference, solely in plasma samples. We fully validated the assay for quality control of the irinotecan solution in the injection dosage form: the standard curve was linear over the concentration range of 0.90 to at least 37.00 μg ml−1. The CV% on all quality control levels was determined to be ≤5.81% for repeatability and ≤6.62% for intermediate precision. Recovery was between 96.5% and 101.9%. Upon comparison with the LC/UV method, we demonstrated very good agreement and acceptable bias between the two methods (slope 0.973, y-intercept 0.0064). Similarly, the technical parameters of the assay in plasma satisfied international guidelines for method validation: the useful analytical range was determined to be between 0.93 and at least 10.00 μg ml−1. This is suitable for quantifying irinotecan in the plasma of treated patients, in the upper region of its therapeutic window, to decide whether dose adjustment is required. Repeatability and intermediate precision (CV%) were within 4.49% and 9.91%, respectively. Recovery was between 96.3% and 103.8%. There was a lack of significant interference by mild hemolysis or by icterus. Irinotecan extraction efficiency from plasma was within 77.9–68.5%. Our results indicated that the proposed method allows quantitative determination of irinotecan plasma levels with acceptable analytical characteristics. The advantages of the proposed method in both matrices, in terms of specificity, rapidity, simplicity, environmental impact and cost effectiveness, are discussed.
中文翻译:

一种简便,快速且低成本的分光光度法,用于定量测定人血浆和药物剂型中的伊立替康
伊立替康是一种抗癌药,有望从个性化给药中受益。因此,快速程序对于监测已治疗患者的伊立替康至关重要。这项工作的目的是开发和验证一种快速简便的可见光分光光度法,用于定量测定药物剂型中的伊立替康,并进一步研究其对血浆中伊立替康分析的有用性。基于所述伊立替康368分之355纳米峰值的在非常低的pH值(0.2)〜400nm的移位,我们建立了线性吸光度之间在400nm处和伊立替康浓度在伊立替康溶液的稀释液注射关系(- [R 2 ≥0.999 )和血浆中含有伊立替康(R 2≥0.995)。仅在血浆样品中,在455 nm处进行背景吸光度校正对于最小化背景干扰至关重要。我们完全验证了注射剂型伊立替康溶液质量控制的测定方法:标准曲线在0.90到至少37.00μgml -1的浓度范围内呈线性。在所有质量控制水平上,CV%的可重复性确定为≤5.81%,中等精度的确定为≤6.62%。回收率在96.5%和101.9%之间。与LC / UV方法进行比较后,我们证明了这两种方法之间的一致性和可接受的偏差(斜率0.973,y-拦截0.0064)。同样,血浆中检测的技术参数满足方法验证的国际准则:有效分析范围确定为0.93至至少10.00μgml -1。这适用于在治疗窗口上部区域对已治疗患者血浆中的伊立替康进行定量,以决定是否需要调整剂量。重复性和中间精度(CV%)分别在4.49%和9.91%之内。回收率在96.3%至103.8%之间。轻度溶血或黄疸缺乏明显的干扰。伊立替康从血浆中的提取效率在77.9–68.5%之内。我们的结果表明,所提出的方法可以定量测定具有可接受分析特性的伊立替康血浆水平。在特异性,快速性,简便性,环境影响和成本效益方面,讨论了该方法在两种基质中的优势。
更新日期:2020-12-24
中文翻译:

一种简便,快速且低成本的分光光度法,用于定量测定人血浆和药物剂型中的伊立替康
伊立替康是一种抗癌药,有望从个性化给药中受益。因此,快速程序对于监测已治疗患者的伊立替康至关重要。这项工作的目的是开发和验证一种快速简便的可见光分光光度法,用于定量测定药物剂型中的伊立替康,并进一步研究其对血浆中伊立替康分析的有用性。基于所述伊立替康368分之355纳米峰值的在非常低的pH值(0.2)〜400nm的移位,我们建立了线性吸光度之间在400nm处和伊立替康浓度在伊立替康溶液的稀释液注射关系(- [R 2 ≥0.999 )和血浆中含有伊立替康(R 2≥0.995)。仅在血浆样品中,在455 nm处进行背景吸光度校正对于最小化背景干扰至关重要。我们完全验证了注射剂型伊立替康溶液质量控制的测定方法:标准曲线在0.90到至少37.00μgml -1的浓度范围内呈线性。在所有质量控制水平上,CV%的可重复性确定为≤5.81%,中等精度的确定为≤6.62%。回收率在96.5%和101.9%之间。与LC / UV方法进行比较后,我们证明了这两种方法之间的一致性和可接受的偏差(斜率0.973,y-拦截0.0064)。同样,血浆中检测的技术参数满足方法验证的国际准则:有效分析范围确定为0.93至至少10.00μgml -1。这适用于在治疗窗口上部区域对已治疗患者血浆中的伊立替康进行定量,以决定是否需要调整剂量。重复性和中间精度(CV%)分别在4.49%和9.91%之内。回收率在96.3%至103.8%之间。轻度溶血或黄疸缺乏明显的干扰。伊立替康从血浆中的提取效率在77.9–68.5%之内。我们的结果表明,所提出的方法可以定量测定具有可接受分析特性的伊立替康血浆水平。在特异性,快速性,简便性,环境影响和成本效益方面,讨论了该方法在两种基质中的优势。









































 京公网安备 11010802027423号
京公网安备 11010802027423号