当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-free, Mild, and Selective Synthesis of Bis(pyrazolyl)alkanes by Nucleophile-Catalyzed Condensation
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-12-24 , DOI: 10.1021/acs.joc.0c02442 Maxym Tansky 1 , Zipeng Gu 1 , Robert J. Comito 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-12-24 , DOI: 10.1021/acs.joc.0c02442 Maxym Tansky 1 , Zipeng Gu 1 , Robert J. Comito 1
Affiliation
|
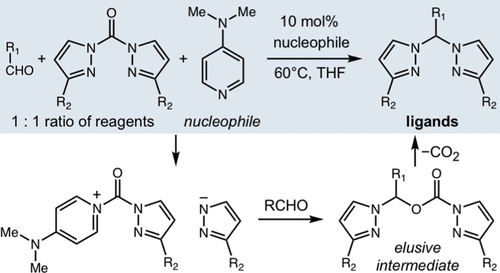
Bis(pyrazolyl)alkanes are a prolific class of ligands for catalysis, accessible by the condensation between bis(pyrazolyl)methanones and carbonyls. In this report, we describe a nucleophile-catalyzed innovation on this condensation that avoids the transition metals, high temperatures, reagent excess, and air-sensitive reagents common among the existing protocols. Significantly, this method accommodates sterically hindered and electronically diverse pyrazoles and aldehydes, applicable for systematic ligand optimization. Furthermore, our scope includes azoles and bridging functional groups previously unreported for this reaction, promising for new heteroscorpionate catalysts. We provide the first direct evidence for an elusive reaction intermediate and characterize the most complete mechanism for this condensation.
中文翻译:

亲核催化剂催化的无金属,轻度和选择性合成双(吡唑基)烷烃
双(吡唑基)烷烃是一类最多的催化配体,可通过双之间的缩合来获得(吡唑基)亚甲基和羰基。在本报告中,我们描述了这种缩合反应的亲核试剂催化创新,避免了现有协议中常见的过渡金属,高温,试剂过量和对空气敏感的试剂。重要的是,该方法可容纳空间受阻和电子形式多样的吡唑和醛,适用于系统的配体优化。此外,我们的研究范围包括以前未报道过的该反应的唑类和桥连官能团,有望用于新型杂蝎子催化剂。我们提供了难以捉摸的反应中间体的第一个直接证据,并描述了这种缩合反应的最完整机理。
更新日期:2021-01-16
中文翻译:

亲核催化剂催化的无金属,轻度和选择性合成双(吡唑基)烷烃
双(吡唑基)烷烃是一类最多的催化配体,可通过双之间的缩合来获得(吡唑基)亚甲基和羰基。在本报告中,我们描述了这种缩合反应的亲核试剂催化创新,避免了现有协议中常见的过渡金属,高温,试剂过量和对空气敏感的试剂。重要的是,该方法可容纳空间受阻和电子形式多样的吡唑和醛,适用于系统的配体优化。此外,我们的研究范围包括以前未报道过的该反应的唑类和桥连官能团,有望用于新型杂蝎子催化剂。我们提供了难以捉摸的反应中间体的第一个直接证据,并描述了这种缩合反应的最完整机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号