当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Cosolvent Ethanol on Solubilization of Ionic Liquids in Supercritical CO2 Microemulsions: Experiments and Simulations
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-12-24 , DOI: 10.1021/acs.jced.0c00725 Hongyue Zhu 1 , Jianzhong Yin 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-12-24 , DOI: 10.1021/acs.jced.0c00725 Hongyue Zhu 1 , Jianzhong Yin 1
Affiliation
|
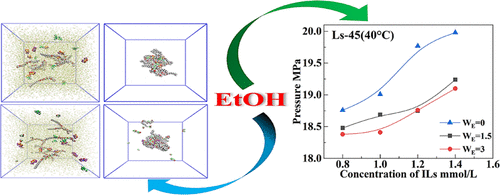
In the presence of ethanol (EtOH), supercritical carbon dioxide (scCO2) microemulsions containing 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([Emim][Tf2N]) ionic liquid (IL) based on nonionic surfactants (Ls-45, Ls-54, Ls-36, Dynol-604, and TMN-6) were constructed. We investigated the solubilization effect of the microemulsion for [Emim][Tf2N] and analyzed the factors that affected the cloud point pressure (CPP), including temperature, IL concentration, and EtOH concentration. The results showed that as the temperature and IL concentration increased, the CPP gradually increased. Besides, small amounts of EtOH could decrease the CPP of the system. Furthermore, the higher the EtOH concentration, the more the CPP decrease. The results indicated that the addition of EtOH was beneficial to the stabilization of the microemulsion. Molecular dynamics (MD) simulations were performed to have an insight into the microstructure of Ls-45/[Emim][Tf2N]/CO2 ternary and EtOH/Ls-45/[Emim][Tf2N]/CO2 quaternary microemulsion systems. The snapshots of the two systems were presented, and the radial distribution function (RDF), the gyration radius (Rg), density distributions, and distance information were calculated to investigate the interfacial structural properties. The dynamic process showed that the self-assembly of the reverse micelle took 87 ns for the ternary system and 84 ns for the quaternary system and remained stable until 100 ns. The formation of microemulsion was proved directly, and the microstructure was briefly analyzed. Finally, the possible structure of the scCO2 microemulsion containing [Emim][Tf2N] and EtOH was inferred.
中文翻译:

助溶剂乙醇对离子液体在超临界CO 2微乳液中增溶的影响:实验和模拟
在乙醇(EtOH)存在下,基于非离子表面活性剂的含有1-乙基-3-甲基咪唑鎓双(三氟甲基磺酰基)酰亚胺([Emim] [Tf 2 N])离子液体(IL)的超临界二氧化碳(scCO 2)微乳液构建了Ls-45,Ls-54,Ls-36,Dynol-604和TMN-6)。我们研究了微乳液对[Emim] [Tf 2 ]的增溶作用N]并分析了影响浊点压力(CPP)的因素,包括温度,IL浓度和EtOH浓度。结果表明,随着温度和IL浓度的升高,CPP逐渐升高。此外,少量的乙醇可能会降低系统的CPP。此外,EtOH浓度越高,CPP下降越多。结果表明,添加EtOH有利于微乳液的稳定。进行分子动力学(MD)模拟以深入了解Ls-45 / [Emim] [Tf 2 N] / CO 2三元和EtOH / Ls-45 / [Emim] [Tf 2 N] / CO 2的微观结构四元微乳液系统。给出了两个系统的快照,并计算了径向分布函数(RDF),回转半径(R g),密度分布和距离信息以研究界面结构特性。动力学过程表明,反胶束的自组装三元体系耗时87 ns,四元体系耗时84 ns,直到100 ns都保持稳定。直接证明了微乳液的形成,并简要分析了其微结构。最后,推断出含有[Emim] [Tf 2 N]和EtOH的scCO 2微乳液的可能结构。
更新日期:2021-01-14
中文翻译:

助溶剂乙醇对离子液体在超临界CO 2微乳液中增溶的影响:实验和模拟
在乙醇(EtOH)存在下,基于非离子表面活性剂的含有1-乙基-3-甲基咪唑鎓双(三氟甲基磺酰基)酰亚胺([Emim] [Tf 2 N])离子液体(IL)的超临界二氧化碳(scCO 2)微乳液构建了Ls-45,Ls-54,Ls-36,Dynol-604和TMN-6)。我们研究了微乳液对[Emim] [Tf 2 ]的增溶作用N]并分析了影响浊点压力(CPP)的因素,包括温度,IL浓度和EtOH浓度。结果表明,随着温度和IL浓度的升高,CPP逐渐升高。此外,少量的乙醇可能会降低系统的CPP。此外,EtOH浓度越高,CPP下降越多。结果表明,添加EtOH有利于微乳液的稳定。进行分子动力学(MD)模拟以深入了解Ls-45 / [Emim] [Tf 2 N] / CO 2三元和EtOH / Ls-45 / [Emim] [Tf 2 N] / CO 2的微观结构四元微乳液系统。给出了两个系统的快照,并计算了径向分布函数(RDF),回转半径(R g),密度分布和距离信息以研究界面结构特性。动力学过程表明,反胶束的自组装三元体系耗时87 ns,四元体系耗时84 ns,直到100 ns都保持稳定。直接证明了微乳液的形成,并简要分析了其微结构。最后,推断出含有[Emim] [Tf 2 N]和EtOH的scCO 2微乳液的可能结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号