当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On-DNA Palladium-Catalyzed Hydrogenation-like Reaction Suitable for DNA-Encoded Library Synthesis
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-12-24 , DOI: 10.1021/acs.bioconjchem.0c00566 Julián Priego 1 , Eduardo de Pedro Beato 1 , Jesús Benavides 1 , Adrián Gironda-Martínez 1 , Fernando González 1 , Jesús Blas 1 , María Dolores Martín-Ortega 1 , Ramón Rama-Garda 1 , Jesús Ezquerra 1 , Miguel A Toledo 1 , Alicia Torrado 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-12-24 , DOI: 10.1021/acs.bioconjchem.0c00566 Julián Priego 1 , Eduardo de Pedro Beato 1 , Jesús Benavides 1 , Adrián Gironda-Martínez 1 , Fernando González 1 , Jesús Blas 1 , María Dolores Martín-Ortega 1 , Ramón Rama-Garda 1 , Jesús Ezquerra 1 , Miguel A Toledo 1 , Alicia Torrado 1
Affiliation
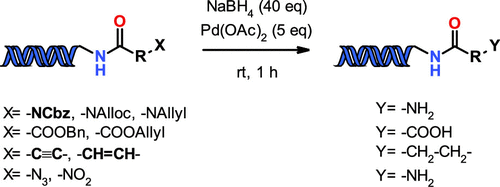
|
Herein we describe a method to orthogonally remove on-DNA N-Cbz, N-Alloc, N-Allyl, O-Bn, and O-Allyl protecting groups in the presence of other common protecting groups to afford free amines and carboxylic acids, respectively. The developed method uses NaBH4 as the source of hydrogen in the presence of Pd(OAc)2 under DNA aqueous conditions. In addition, under the developed conditions we were able to successfully hydrogenate triple and double bonds to totally saturated compounds. Furthermore, we introduce a new alternative procedure to reduce azides and aromatic nitro compounds to primary amines.
中文翻译:

适用于 DNA 编码文库合成的 On-DNA 钯催化类氢化反应
本文中,我们描述到正交去除-DNA的方法Ñ -Cbz,Ñ -alloc,Ñ分别烯丙基,O- BN,和O-烯丙基的保护基团中其它常见的保护基团,得到游离胺和羧酸的存在下, . 所开发的方法在 DNA 水溶液条件下,在 Pd(OAc) 2存在下,使用 NaBH 4作为氢源。此外,在开发的条件下,我们能够成功地将三键和双键氢化成完全饱和的化合物。此外,我们引入了一种新的替代方法,将叠氮化物和芳香族硝基化合物还原为伯胺。
更新日期:2021-01-20
中文翻译:

适用于 DNA 编码文库合成的 On-DNA 钯催化类氢化反应
本文中,我们描述到正交去除-DNA的方法Ñ -Cbz,Ñ -alloc,Ñ分别烯丙基,O- BN,和O-烯丙基的保护基团中其它常见的保护基团,得到游离胺和羧酸的存在下, . 所开发的方法在 DNA 水溶液条件下,在 Pd(OAc) 2存在下,使用 NaBH 4作为氢源。此外,在开发的条件下,我们能够成功地将三键和双键氢化成完全饱和的化合物。此外,我们引入了一种新的替代方法,将叠氮化物和芳香族硝基化合物还原为伯胺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号