当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cu-Modified SrTiO3 Perovskites Toward Enhanced Water–Gas Shift Catalysis: A Combined Experimental and Computational Study
ACS Applied Energy Materials ( IF 6.4 ) Pub Date : 2020-12-23 , DOI: 10.1021/acsaem.0c02371 Vitor C. Coletta 1 , Renato V. Gonçalves 1 , Maria I. B. Bernardi 1 , Dorian A. H. Hanaor 2 , M. Hussein N. Assadi 3 , Francielle C. F. Marcos 4 , Francisco G. E. Nogueira 5 , Elisabete M. Assaf 6 , Valmor R. Mastelaro 1
ACS Applied Energy Materials ( IF 6.4 ) Pub Date : 2020-12-23 , DOI: 10.1021/acsaem.0c02371 Vitor C. Coletta 1 , Renato V. Gonçalves 1 , Maria I. B. Bernardi 1 , Dorian A. H. Hanaor 2 , M. Hussein N. Assadi 3 , Francielle C. F. Marcos 4 , Francisco G. E. Nogueira 5 , Elisabete M. Assaf 6 , Valmor R. Mastelaro 1
Affiliation
|
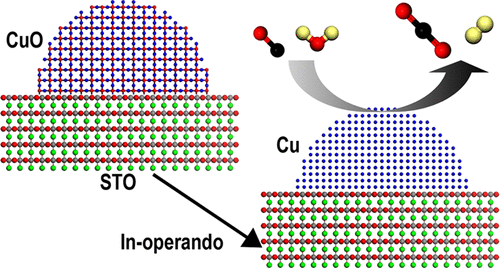
A water–gas shift reaction (WGS) is important and widely applied in the production of H2. Cu-modified perovskites are promising catalysts for WGS reactions in hydrogen generation. However, the structure-dependent stability and reaction pathways of such materials remain unclear. Herein, we report catalytically active Cu-modified SrTiO3 (nominally SrTi1–xCuxO3) prepared by a modified polymeric precursor method. Microstructural analysis revealed a partially segregated CuO phase in the as-prepared materials. Operando X-ray diffraction and absorption spectroscopy showed the reduction of CuO into a stable metallic phase under conditions of WGS reactions for all compositions. Among the characterized materials, the x = 0.20 composition showed the highest turnover frequency, lowest activation energy, and the highest WGS rate at 300 °C. According to density functional calculations, the formation of CuO is energetically less favorable compared with SrTiO3, explaining why the segregated CuO phase on the SrTiO3 surface is reduced to Cu during the catalytic reaction, while SrTiO3 remains. For x = 0.20, the size of the segregated CuO phase is optimum for facilitating the catalytic reaction. In contrast, a higher Cu content (x = 0.3) results in an aggregation of smaller CuO particles, resulting in fewer surface active sites and a net decrease in catalytic performance.
中文翻译:

Cu改性SrTiO 3钙钛矿对增强水煤气变换催化的实验和计算研究相结合
水煤气变换反应(WGS)很重要,并广泛应用于H 2的生产中。铜改性钙钛矿是有希望的催化剂,可用于制氢的WGS反应。但是,这种材料的结构依赖性稳定性和反应途径仍然不清楚。在此,我们报告了具有催化活性的铜改性SrTiO 3(名义上为SrTi 1– x Cu x O 3)通过改进的聚合前体方法制备。显微组织分析表明,所制备的材料中CuO相部分偏析。Operando X射线衍射和吸收光谱表明,在所有组合物的WGS反应条件下,CuO还原为稳定的金属相。在表征的材料中,x = 0.20的成分在300°C时表现出最高的转换频率,最低的活化能和最高的WGS速率。根据密度泛函计算,与SrTiO 3相比,CuO的形成在能量上不利,这解释了为什么SrTiO 3表面的偏析CuO相在催化反应过程中被还原为Cu,而SrTiO 3保留了下来。对于x= 0.20,偏析的CuO相的尺寸对于促进催化反应是最佳的。相反,较高的Cu含量(x = 0.3)导致较小的CuO颗粒聚集,从而导致较少的表面活性位点和催化性能的净降低。
更新日期:2021-01-25
中文翻译:

Cu改性SrTiO 3钙钛矿对增强水煤气变换催化的实验和计算研究相结合
水煤气变换反应(WGS)很重要,并广泛应用于H 2的生产中。铜改性钙钛矿是有希望的催化剂,可用于制氢的WGS反应。但是,这种材料的结构依赖性稳定性和反应途径仍然不清楚。在此,我们报告了具有催化活性的铜改性SrTiO 3(名义上为SrTi 1– x Cu x O 3)通过改进的聚合前体方法制备。显微组织分析表明,所制备的材料中CuO相部分偏析。Operando X射线衍射和吸收光谱表明,在所有组合物的WGS反应条件下,CuO还原为稳定的金属相。在表征的材料中,x = 0.20的成分在300°C时表现出最高的转换频率,最低的活化能和最高的WGS速率。根据密度泛函计算,与SrTiO 3相比,CuO的形成在能量上不利,这解释了为什么SrTiO 3表面的偏析CuO相在催化反应过程中被还原为Cu,而SrTiO 3保留了下来。对于x= 0.20,偏析的CuO相的尺寸对于促进催化反应是最佳的。相反,较高的Cu含量(x = 0.3)导致较小的CuO颗粒聚集,从而导致较少的表面活性位点和催化性能的净降低。



























 京公网安备 11010802027423号
京公网安备 11010802027423号