Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-12-18 , DOI: 10.1016/j.tetlet.2020.152734 Wen-Qing Zhu , Jin Zhang , Pan Fan , Lan-Ting Shi , Hong Li , Min-Ge Yang , Yang Li
|
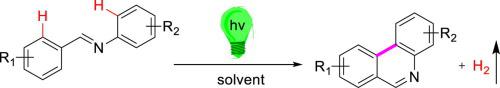
A new method for synthesizing phenanthridines by photocyclization has been established. This method does not require inert gas protection, does not require transition metal catalysts and is environmentally friendly, efficient and convenient. It is proposed to use (E)-N,1-diphenylformimines as substrates to synthesize phenanthridine and its derivatives by ultraviolet light, which provides a new synthesis route for further research on the synthesis of phenanthridines by photocyclization. Eight new phenanthridine compounds were synthesized. The confirmation of their structures provides a material basis for further study of their properties and tapping of their potential for applications. The establishment of this method further broadens the synthetic pathways of phenanthridine compounds.
中文翻译:

直接脱氢条件下菲啶及其衍生物的光环合成
建立了一种通过光环化合成菲啶的新方法。该方法不需要惰性气体保护,不需要过渡金属催化剂,并且是环境友好,有效和方便的。提出以(E)-N,1-二苯基甲亚胺为底物通过紫外光合成菲啶及其衍生物,为进一步研究光环化合成菲啶提供了一条新的合成途径。合成了八种新的菲啶化合物。其结构的确认为进一步研究其性能和挖掘其应用潜力提供了物质基础。该方法的建立进一步拓宽了菲啶化合物的合成途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号