Molecular Metabolism ( IF 7.0 ) Pub Date : 2020-12-24 , DOI: 10.1016/j.molmet.2020.101157 Justin P Hardee 1 , Karen J B Martins 1 , Paula M Miotto 2 , James G Ryall 1 , Stefan M Gehrig 1 , Boris Reljic 3 , Timur Naim 1 , Jin D Chung 1 , Jen Trieu 1 , Kristy Swiderski 1 , Ashleigh M Philp 4 , Andrew Philp 4 , Matthew J Watt 2 , David A Stroud 3 , Rene Koopman 1 , Gregory R Steinberg 5 , Gordon S Lynch 1
|
Objectives
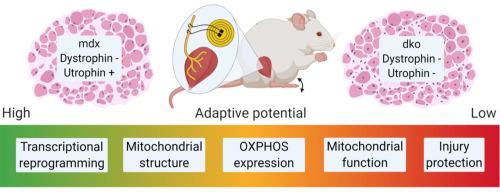
Preferential damage to fast, glycolytic myofibers is common in many muscle-wasting diseases, including Duchenne muscular dystrophy (DMD). Promoting an oxidative phenotype could protect muscles from damage and ameliorate the dystrophic pathology with therapeutic relevance, but developing efficacious strategies requires understanding currently unknown biological roles for dystrophin and utrophin in dystrophic muscle adaptation and plasticity.
Methods
Combining whole transcriptome RNA sequencing and mitochondrial proteomics with assessments of metabolic and contractile function, we investigated the roles of dystrophin and utrophin in fast-to-slow muscle remodeling with low-frequency electrical stimulation (LFS, 10 Hz, 12 h/d, 7 d/wk, 28 d) in mdx (dystrophin null) and dko (dystrophin/utrophin null) mice, two established preclinical models of DMD.
Results
Novel biological roles in adaptation were demonstrated by impaired transcriptional activation of estrogen-related receptor alpha-responsive genes supporting oxidative phosphorylation in dystrophic muscles. Further, utrophin expression in dystrophic muscles was required for LFS-induced remodeling of mitochondrial respiratory chain complexes, enhanced fiber respiration, and conferred protection from eccentric contraction-mediated damage.
Conclusions
These findings reveal novel roles for dystrophin and utrophin during LFS-induced metabolic remodeling of dystrophic muscle and highlight the therapeutic potential of LFS to ameliorate the dystrophic pathology and protect from contraction-induced injury with important implications for DMD and related muscle disorders.
中文翻译:

营养不良骨骼肌的代谢重塑揭示了抗肌萎缩蛋白和优营养蛋白在适应和可塑性中的生物学作用
目标
对快速糖酵解肌纤维的优先损伤在许多肌肉萎缩疾病中很常见,包括杜氏肌营养不良症 (DMD)。促进氧化表型可以保护肌肉免受损伤并改善具有治疗相关性的营养不良病理,但制定有效的策略需要了解目前未知的肌营养不良蛋白和 utrophin 在营养不良肌肉适应和可塑性中的生物学作用。
方法
将全转录组 RNA 测序和线粒体蛋白质组学与代谢和收缩功能的评估相结合,我们研究了肌营养不良蛋白和 utrophin 在低频电刺激(LFS,10 Hz,12 h/d,7 d/wk, 28 d) 在mdx(无肌养蛋白)和dko(无肌养蛋白/无肌养蛋白)小鼠中,两个已建立的 DMD 临床前模型。
结果
支持营养不良肌肉中氧化磷酸化的雌激素相关受体 α 反应基因的转录激活受损,证明了在适应中新的生物学作用。此外,LFS 诱导的线粒体呼吸链复合物重塑、增强纤维呼吸和保护免受离心收缩介导的损伤,需要营养不良肌肉中的 utrophin 表达。
结论
这些发现揭示了肌营养不良蛋白和 utrophin 在 LFS 诱导的营养不良肌肉代谢重塑过程中的新作用,并突出了 LFS 改善营养不良病理和防止收缩引起的损伤的治疗潜力,对 DMD 和相关肌肉疾病具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号