当前位置:
X-MOL 学术
›
RSC Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mapping the energy landscape of protein–ligand binding via linear free energy relationships determined by protein NMR relaxation dispersion
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2020-12-23 , DOI: 10.1039/d0cb00229a Olof Stenström 1 , Carl Diehl 1 , Kristofer Modig 1 , Ulf J Nilsson 2 , Mikael Akke 1
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2020-12-23 , DOI: 10.1039/d0cb00229a Olof Stenström 1 , Carl Diehl 1 , Kristofer Modig 1 , Ulf J Nilsson 2 , Mikael Akke 1
Affiliation
|
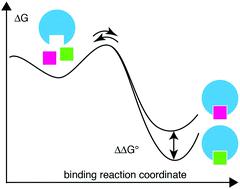
Biochemical signaling is mediated by complexes between macromolecular receptors and their ligands, with the duration of the signal being directly related to the lifetime of the ligand–receptor complex. In the field of drug design, the recognition that drug efficacy in vivo depends on the lifetime of the drug–protein complex has spawned the concept of designing drugs with particular binding kinetics. To advance this field it is critical to investigate how the molecular details of designed ligands might affect the binding kinetics, as well as the equilibrium binding constant. Here we use protein NMR relaxation dispersion to determine linear free energy relationships involving the on- and off-rates and the affinity for a series of congeneric ligands targeting the carbohydrate recognition domain of galectin-3. Using this approach we determine the energy landscape and the position of the transition state along the reaction coordinate of protein–ligand binding. The results show that ligands exhibiting reduced off-rates achieve this by primarily stabilizing the bound state, but do not affect the transition state to any greater extent. The transition state forms early, that is, it is located significantly closer to the free state than to the bound state, suggesting a critical role of desolvation. Furthermore, the data suggest that different subclasses of ligands show different behavior with respect to these characteristics.
中文翻译:

通过蛋白质 NMR 弛豫色散确定的线性自由能关系绘制蛋白质-配体结合的能量图
生化信号由大分子受体与其配体之间的复合物介导,信号的持续时间与配体-受体复合物的寿命直接相关。在药物设计领域,人们认识到体内药物功效取决于药物-蛋白质复合物的寿命,从而催生了设计具有特定结合动力学的药物的概念。为了推进这一领域的发展,研究设计配体的分子细节如何影响结合动力学以及平衡结合常数至关重要。在这里,我们使用蛋白质 NMR 弛豫色散来确定涉及结合速率和解离速率的线性自由能关系以及针对半乳糖凝集素 3 碳水化合物识别域的一系列同源配体的亲和力。使用这种方法,我们确定了沿着蛋白质-配体结合的反应坐标的能量格局和过渡态的位置。结果表明,解离速率降低的配体主要通过稳定结合态来实现这一点,但不会在更大程度上影响过渡态。过渡态形成较早,也就是说,它的位置比束缚态更接近自由态,这表明去溶剂化的关键作用。此外,数据表明配体的不同亚类在这些特征方面表现出不同的行为。
更新日期:2020-12-23
中文翻译:

通过蛋白质 NMR 弛豫色散确定的线性自由能关系绘制蛋白质-配体结合的能量图
生化信号由大分子受体与其配体之间的复合物介导,信号的持续时间与配体-受体复合物的寿命直接相关。在药物设计领域,人们认识到体内药物功效取决于药物-蛋白质复合物的寿命,从而催生了设计具有特定结合动力学的药物的概念。为了推进这一领域的发展,研究设计配体的分子细节如何影响结合动力学以及平衡结合常数至关重要。在这里,我们使用蛋白质 NMR 弛豫色散来确定涉及结合速率和解离速率的线性自由能关系以及针对半乳糖凝集素 3 碳水化合物识别域的一系列同源配体的亲和力。使用这种方法,我们确定了沿着蛋白质-配体结合的反应坐标的能量格局和过渡态的位置。结果表明,解离速率降低的配体主要通过稳定结合态来实现这一点,但不会在更大程度上影响过渡态。过渡态形成较早,也就是说,它的位置比束缚态更接近自由态,这表明去溶剂化的关键作用。此外,数据表明配体的不同亚类在这些特征方面表现出不同的行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号