当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Systematic Analysis of Protein Targets Associated with Adverse Events of Drugs from Clinical Trials and Postmarketing Reports
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-12-22 , DOI: 10.1021/acs.chemrestox.0c00294 Ines A Smit 1 , Avid M Afzal 1 , Chad H G Allen 1 , Fredrik Svensson 1 , Thierry Hanser 2 , Andreas Bender 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-12-22 , DOI: 10.1021/acs.chemrestox.0c00294 Ines A Smit 1 , Avid M Afzal 1 , Chad H G Allen 1 , Fredrik Svensson 1 , Thierry Hanser 2 , Andreas Bender 1
Affiliation
|
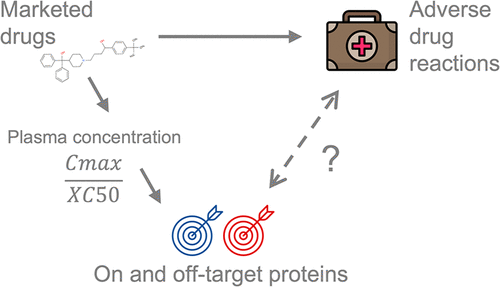
Adverse drug reactions (ADRs) are undesired effects of medicines that can harm patients and are a significant source of attrition in drug development. ADRs are anticipated by routinely screening drugs against secondary pharmacology protein panels. However, there is still a lack of quantitative information on the links between these off-target proteins and the reporting of ADRs in humans. Here, we present a systematic analysis of associations between measured and predicted in vitro bioactivities of drugs and adverse events (AEs) in humans from two sources of data: the Side Effect Resource, derived from clinical trials, and the Food and Drug Administration Adverse Event Reporting System, derived from postmarketing surveillance. The ratio of a drug’s therapeutic unbound plasma concentration over the drug’s in vitro potency against a given protein was used to select proteins most likely to be relevant to in vivo effects. In examining individual target bioactivities as predictors of AEs, we found a trade-off between the positive predictive value and the fraction of drugs with AEs that can be detected. However, considering sets of multiple targets for the same AE can help identify a greater fraction of AE-associated drugs. Of the 45 targets with statistically significant associations to AEs, 30 are included on existing safety target panels. The remaining 15 targets include 9 carbonic anhydrases, of which CA5B is significantly associated with cholestatic jaundice. We include the full quantitative data on associations between measured and predicted in vitro bioactivities and AEs in humans in this work, which can be used to make a more informed selection of safety profiling targets.
中文翻译:

来自临床试验和上市后报告的与药物不良事件相关的蛋白质靶点的系统分析
药物不良反应 (ADR) 是药物的不良反应,会伤害患者,是药物开发过程中损耗的重要来源。通过针对二级药理学蛋白质组常规筛选药物来预测 ADR。然而,仍然缺乏关于这些脱靶蛋白与人类 ADR 报告之间联系的定量信息。在这里,我们从两个数据来源对药物的测量和预测的体外生物活性与人体不良事件 (AE)之间的关联进行系统分析:来自临床试验的副作用资源和美国食品和药物管理局的不良事件报告系统,源自上市后监督。药物的治疗性未结合血浆浓度与药物浓度之比针对给定蛋白质的体外效力用于选择最可能与体内效应相关的蛋白质。在检查作为 AE 预测因子的个体目标生物活性时,我们发现了阳性预测值与可检测到的具有 AE 的药物比例之间的权衡。然而,考虑同一 AE 的多个目标集可以帮助识别更多的 AE 相关药物。在与 AE 具有统计学显着关联的 45 个目标中,30 个包含在现有的安全目标面板中。其余 15 个靶标包括 9 个碳酸酐酶,其中 CA5B 与胆汁淤积性黄疸显着相关。我们包括关于体外测量和预测之间关联的完整定量数据 人类在这项工作中的生物活性和 AE,可用于更明智地选择安全分析目标。
更新日期:2021-02-15
中文翻译:

来自临床试验和上市后报告的与药物不良事件相关的蛋白质靶点的系统分析
药物不良反应 (ADR) 是药物的不良反应,会伤害患者,是药物开发过程中损耗的重要来源。通过针对二级药理学蛋白质组常规筛选药物来预测 ADR。然而,仍然缺乏关于这些脱靶蛋白与人类 ADR 报告之间联系的定量信息。在这里,我们从两个数据来源对药物的测量和预测的体外生物活性与人体不良事件 (AE)之间的关联进行系统分析:来自临床试验的副作用资源和美国食品和药物管理局的不良事件报告系统,源自上市后监督。药物的治疗性未结合血浆浓度与药物浓度之比针对给定蛋白质的体外效力用于选择最可能与体内效应相关的蛋白质。在检查作为 AE 预测因子的个体目标生物活性时,我们发现了阳性预测值与可检测到的具有 AE 的药物比例之间的权衡。然而,考虑同一 AE 的多个目标集可以帮助识别更多的 AE 相关药物。在与 AE 具有统计学显着关联的 45 个目标中,30 个包含在现有的安全目标面板中。其余 15 个靶标包括 9 个碳酸酐酶,其中 CA5B 与胆汁淤积性黄疸显着相关。我们包括关于体外测量和预测之间关联的完整定量数据 人类在这项工作中的生物活性和 AE,可用于更明智地选择安全分析目标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号