当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MRGBP, a member of the NuA4 complex, inhibits DNA double‐strand break repair
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-12-22 , DOI: 10.1002/2211-5463.13071 Sabrina Rivero 1, 2 , Guillermo Rodríguez-Real 2, 3 , Inés Marín 2 , Pablo Huertas 2, 3
FEBS Open Bio ( IF 2.8 ) Pub Date : 2020-12-22 , DOI: 10.1002/2211-5463.13071 Sabrina Rivero 1, 2 , Guillermo Rodríguez-Real 2, 3 , Inés Marín 2 , Pablo Huertas 2, 3
Affiliation
|
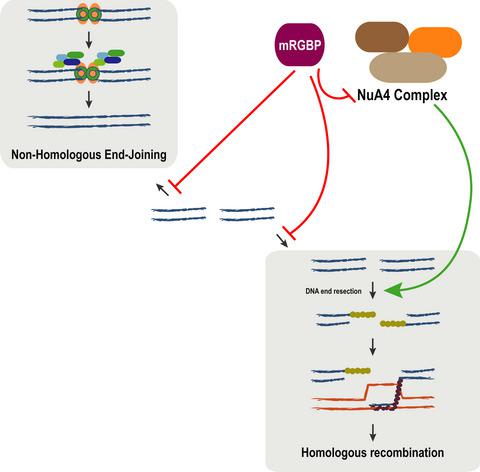
The repair of DNA breaks takes place in the context of chromatin and thus involves the activity of chromatin remodelers. The nucleosome acetyltransferase of H4 (NuA4) remodeler complex enables DNA break repair by relaxing flanking chromatin. Here, we show that MRG domain binding protein (MRGBP), a member of this complex, acts as a general inhibitor of DNA double‐strand break repair. Upon its downregulation, repair is generally increased. This is particularly evident for the stimulation of early events of homologous recombination. Thus, MRGBP has an opposing role to the main catalytic subunits of the NuA4 complex. Our data suggest that MRGBP acts by limiting the activity of this complex in DNA repair, specifically by narrowing the extent of DNA‐end resection.
中文翻译:

MRBP,NuA4 复合物的成员,抑制 DNA 双链断裂修复
DNA 断裂的修复发生在染色质的背景下,因此涉及染色质重塑者的活动。H4 (NuA4) 重塑复合物的核小体乙酰转移酶通过放松侧翼染色质来实现 DNA 断裂修复。在这里,我们展示了 MRG 域结合蛋白 (MRGP),该复合物的一个成员,作为 DNA 双链断裂修复的通用抑制剂。在下调时,修复通常会增加。这对于刺激同源重组的早期事件尤其明显。因此,MRGBP 与 NuA4 复合物的主要催化亚基具有相反的作用。我们的数据表明,MRGBP 通过限制该复合物在 DNA 修复中的活性发挥作用,特别是通过缩小 DNA 末端切除的范围。
更新日期:2020-12-22
中文翻译:

MRBP,NuA4 复合物的成员,抑制 DNA 双链断裂修复
DNA 断裂的修复发生在染色质的背景下,因此涉及染色质重塑者的活动。H4 (NuA4) 重塑复合物的核小体乙酰转移酶通过放松侧翼染色质来实现 DNA 断裂修复。在这里,我们展示了 MRG 域结合蛋白 (MRGP),该复合物的一个成员,作为 DNA 双链断裂修复的通用抑制剂。在下调时,修复通常会增加。这对于刺激同源重组的早期事件尤其明显。因此,MRGBP 与 NuA4 复合物的主要催化亚基具有相反的作用。我们的数据表明,MRGBP 通过限制该复合物在 DNA 修复中的活性发挥作用,特别是通过缩小 DNA 末端切除的范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号