当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Affinity-based proteomics reveals novel targets of inositol pyrophosphate (5-IP7)-dependent phosphorylation and binding in Trypanosoma cruzi replicative stages
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-12-22 , DOI: 10.1111/mmi.14672 Brian S Mantilla 1, 2 , Karunakaran Kalesh 3 , Nathaniel W Brown 4, 5 , Dorothea Fiedler 4, 6 , Roberto Docampo 1
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-12-22 , DOI: 10.1111/mmi.14672 Brian S Mantilla 1, 2 , Karunakaran Kalesh 3 , Nathaniel W Brown 4, 5 , Dorothea Fiedler 4, 6 , Roberto Docampo 1
Affiliation
|
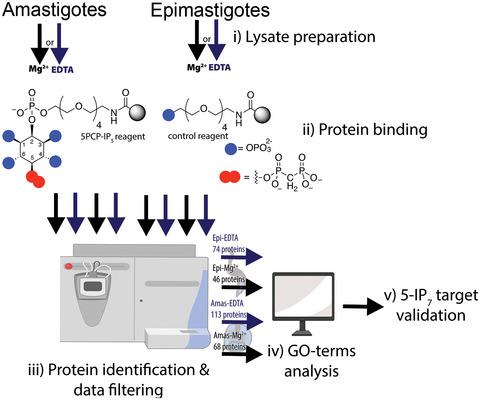
Diphosphoinositol-5-pentakisphosphate (5-PP-IP5), also known as inositol heptakisphosphate (5-IP7), has been described as a high-energy phosphate metabolite that participates in the regulation of multiple cellular processes through protein binding or serine pyrophosphorylation, a posttranslational modification involving a β-phosphoryl transfer. In this study, utilizing an immobilized 5-IP7 affinity reagent, we performed pull-down experiments coupled with mass spectrometry identification, and bioinformatic analysis, to reveal 5-IP7-regulated processes in the two proliferative stages of the unicellular parasite Trypanosoma cruzi. Our protein screen clearly defined two cohorts of putative targets either in the presence of magnesium ions or in metal-free conditions. We endogenously tagged four protein candidates and immunopurified them to assess whether 5-IP7-driven phosphorylation is conserved in T. cruzi. Among the most interesting targets, we identified a choline/o-acetyltransferase domain-containing phosphoprotein that undergoes 5-IP7-mediated phosphorylation events at a polyserine tract (Ser578-580). We also identified a novel SPX domain-containing phosphoribosyltransferase [EC 2.7.6.1] herein termed as TcPRPPS4. Our data revealed new possible functional roles of 5-IP7 in this divergent eukaryote, and provided potential new targets for chemotherapy.
中文翻译:

基于亲和力的蛋白质组学揭示了克氏锥虫复制阶段肌醇焦磷酸 (5-IP7) 依赖性磷酸化和结合的新靶标
Diphosphoinositol-5-pentakisphosphate (5-PP-IP 5 ),也称为肌醇七磷酸盐 (5-IP 7 ),被描述为一种高能磷酸盐代谢物,通过蛋白质结合或丝氨酸参与多种细胞过程的调节焦磷酸化,一种涉及 β-磷酰基转移的翻译后修饰。在这项研究中,我们利用固定化的 5-IP 7亲和试剂,结合质谱鉴定和生物信息学分析进行了 pull-down 实验,以揭示单细胞寄生虫克氏锥虫的两个增殖阶段中5-IP 7的调节过程. 我们的蛋白质筛选清楚地定义了两组假定目标,无论是在镁离子存在下还是在无金属条件下。我们对四种候选蛋白质进行了内源性标记,并对它们进行了免疫纯化,以评估 5-IP 7驱动的磷酸化在克氏锥虫中是否保守。在最有趣的目标中,我们发现了一种包含胆碱/邻乙酰转移酶结构域的磷蛋白,它在聚丝氨酸束 (Ser 578-580 ) 处经历 5-IP 7介导的磷酸化事件。我们还鉴定了一种新的含有 SPX 结构域的磷酸核糖基转移酶 [EC 2.7.6.1],在此称为 TcPRPPS4。我们的数据揭示了 5-IP 7的新功能在这种不同的真核生物中,为化疗提供了潜在的新靶点。
更新日期:2020-12-22
中文翻译:

基于亲和力的蛋白质组学揭示了克氏锥虫复制阶段肌醇焦磷酸 (5-IP7) 依赖性磷酸化和结合的新靶标
Diphosphoinositol-5-pentakisphosphate (5-PP-IP 5 ),也称为肌醇七磷酸盐 (5-IP 7 ),被描述为一种高能磷酸盐代谢物,通过蛋白质结合或丝氨酸参与多种细胞过程的调节焦磷酸化,一种涉及 β-磷酰基转移的翻译后修饰。在这项研究中,我们利用固定化的 5-IP 7亲和试剂,结合质谱鉴定和生物信息学分析进行了 pull-down 实验,以揭示单细胞寄生虫克氏锥虫的两个增殖阶段中5-IP 7的调节过程. 我们的蛋白质筛选清楚地定义了两组假定目标,无论是在镁离子存在下还是在无金属条件下。我们对四种候选蛋白质进行了内源性标记,并对它们进行了免疫纯化,以评估 5-IP 7驱动的磷酸化在克氏锥虫中是否保守。在最有趣的目标中,我们发现了一种包含胆碱/邻乙酰转移酶结构域的磷蛋白,它在聚丝氨酸束 (Ser 578-580 ) 处经历 5-IP 7介导的磷酸化事件。我们还鉴定了一种新的含有 SPX 结构域的磷酸核糖基转移酶 [EC 2.7.6.1],在此称为 TcPRPPS4。我们的数据揭示了 5-IP 7的新功能在这种不同的真核生物中,为化疗提供了潜在的新靶点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号