当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of 3‐Pyrrolines through an Aryne‐Induced Domino Process
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-12-22 , DOI: 10.1002/ajoc.202000711 Jih Ru Hwu, Avijit Panja, Nitesh K. Gupta, Wen‐Chieh Huang, Yu‐Chen Hu, Chun‐Cheng Lin, Kuo‐Chu Hwang, Wei‐Jen Chan, Shwu‐Chen Tsay
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-12-22 , DOI: 10.1002/ajoc.202000711 Jih Ru Hwu, Avijit Panja, Nitesh K. Gupta, Wen‐Chieh Huang, Yu‐Chen Hu, Chun‐Cheng Lin, Kuo‐Chu Hwang, Wei‐Jen Chan, Shwu‐Chen Tsay
|
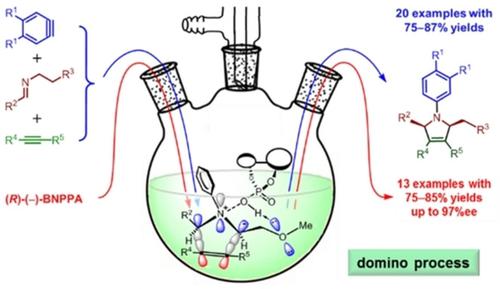
Production of chiral compounds by green processes like domino reactions is of importance to environmental protection and sustainable development. Nevertheless, finding an appropriate catalyst to control enantioselectivity with satisfaction is a major challenge. Here, we report the accomplishment of a newly developed domino reaction for synthesizing optically active 3‐pyrrolines, a class of compounds with various biological properties. The reaction involves the use of (trimethylsilyl)aryl triflates, Schiff bases, and alkynes in the presence of a chiral catalyst (R)‐(−)‐1,1′‐binaphthyl‐2,2′‐diyl hydrogenphosphate. The key features of this new reaction include the generation of a single product in very good yields (75–85%) with high stereo‐ and enantio‐selectivity; the enantiomeric ratio reaches as high as 98.5 : 1.5. Moreover, it involves an aryne‐induced domino reaction and an unusual 1,4‐intramolecular proton transfer, which overwhelms the well‐established 1,5‐proton transfer.
中文翻译:

通过Aryne诱导的Domino过程不对称合成3-吡咯啉
通过多米诺反应等绿色过程生产手性化合物对环境保护和可持续发展具有重要意义。然而,找到令人满意的催化剂来令人满意地控制对映选择性是一个重大挑战。在这里,我们报告了用于合成旋光性3-吡咯啉(一种具有多种生物学特性的化合物)的新开发的多米诺反应的完成情况。该反应涉及在手性催化剂的存在下使用(三甲基甲硅烷基)芳基三氟甲磺酸酯,席夫碱和炔烃(R)-(-)-1,1'-联萘-2-2,2'-磷酸二氢酯。该新反应的关键特征包括以高产率(75-85%)生成具有高立体选择性和对映选择性的单一产品。对映体比率高达98.5:1.5。此外,它涉及由芳烃引起的多米诺骨牌反应和不寻常的1,4分子内质子转移,这压倒了公认的1,5质子转移。
更新日期:2020-12-22
中文翻译:

通过Aryne诱导的Domino过程不对称合成3-吡咯啉
通过多米诺反应等绿色过程生产手性化合物对环境保护和可持续发展具有重要意义。然而,找到令人满意的催化剂来令人满意地控制对映选择性是一个重大挑战。在这里,我们报告了用于合成旋光性3-吡咯啉(一种具有多种生物学特性的化合物)的新开发的多米诺反应的完成情况。该反应涉及在手性催化剂的存在下使用(三甲基甲硅烷基)芳基三氟甲磺酸酯,席夫碱和炔烃(R)-(-)-1,1'-联萘-2-2,2'-磷酸二氢酯。该新反应的关键特征包括以高产率(75-85%)生成具有高立体选择性和对映选择性的单一产品。对映体比率高达98.5:1.5。此外,它涉及由芳烃引起的多米诺骨牌反应和不寻常的1,4分子内质子转移,这压倒了公认的1,5质子转移。



























 京公网安备 11010802027423号
京公网安备 11010802027423号