Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2020-12-23 , DOI: 10.1016/j.jmgm.2020.107826 Suchetana Gupta 1 , Sangeetha Balasubramanian 1 , Sanjib Senapati 1
|
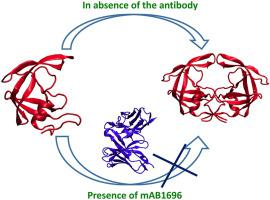
HIV-1 protease is an essential enzyme in the life cycle of human immunodeficiency virus (HIV) and hence is one of the most important targets for antiviral drug design. Although there are ten FDA approved drugs against HIV protease (PR), their long term usage elicits mutations leading to drug resistance. As a result, novel therapeutic approaches are being explored including synthetic antibodies. Recently, a murine monoclonal antibody, mAB1696 (mAB) was reported to inhibit PR by preventing dimerization. Crystallographic data could reveal only six protease residues that interact with mAB. The present study employs a range of computational techniques, starting from protein-protein docking to all-atomic molecular dynamics simulations to generate plausible 3D structures of PR-mAB complex. Results show that mAB interacts very strongly with several PR dimer interface residues, such as Gln7, Arg8 (N-terminal), Cys95, Leu97 (C-terminal), Thr26, Gly27 (active site), Gly49, Ile50 (flap), apart from its interactions with the PR epitope region, Pro1-Trp6 (N-terminal). These observations support the hypothesis that binding of mAB prevents the dimerization of PR. The interactions and binding conformations identified in this study could form the basis for designing allosteric inhibitors preventing the dimerization of HIV-1 Protease.
中文翻译:

了解单克隆抗体抑制HIV-1蛋白酶的机制
HIV-1蛋白酶是人类免疫缺陷病毒(HIV)生命周期中必不可少的酶,因此是抗病毒药物设计的最重要目标之一。尽管有十种经FDA批准的抗HIV蛋白酶(PR)的药物,但它们的长期使用会引起突变,从而导致耐药性。结果,正在探索包括合成抗体在内的新型治疗方法。最近,据报道鼠单克隆抗体mAB1696(mAB)通过阻止二聚化来抑制PR。晶体学数据可以揭示仅六个与mAB相互作用的蛋白酶残基。本研究采用了一系列计算技术,从蛋白质-蛋白质对接到全原子分子动力学模拟,以生成合理的PR-mAB复合物3D结构。结果显示,mAB与几个PR二聚体界面残基(如Gln7,Arg8(N端),Cys95,Leu97(C端),Thr26,Gly27(活性位点),Gly49,Ile50(襟翼))相互作用非常强。从其与PR表位区域Pro1-Trp6(N端)的相互作用中获悉。这些观察结果支持mAB结合阻止PR二聚化的假说。在这项研究中确定的相互作用和结合构象可以为设计防止HIV-1蛋白酶二聚化的变构抑制剂奠定基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号