当前位置:
X-MOL 学术
›
Comput. Struct. Biotechnol. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The impact of Gag non-cleavage site mutations on HIV-1 viral fitness from integrative modelling and simulations
Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2020-12-23 , DOI: 10.1016/j.csbj.2020.12.022 Firdaus Samsudin 1 , Samuel Ken-En Gan 1, 2, 3 , Peter J Bond 1, 4
Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2020-12-23 , DOI: 10.1016/j.csbj.2020.12.022 Firdaus Samsudin 1 , Samuel Ken-En Gan 1, 2, 3 , Peter J Bond 1, 4
Affiliation
|
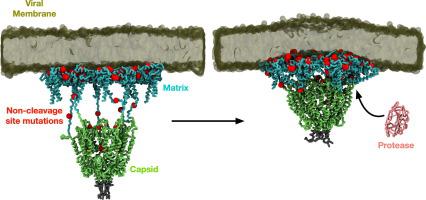
The high mutation rate in retroviruses is one of the leading causes of drug resistance. In human immunodeficiency virus type-1 (HIV-1), synergistic mutations in its protease and the protease substrate – the Group-specific antigen (Gag) polyprotein – work together to confer drug resistance against protease inhibitors and compensate the mutations affecting viral fitness. Some Gag mutations can restore Gag-protease binding, yet most Gag-protease correlated mutations occur outside of the Gag cleavage site. To investigate the molecular basis for this, we now report multiscale modelling approaches to investigate various sequentially cleaved Gag products in the context of clinically relevant mutations that occur outside of the cleavage sites, including simulations of the largest Gag proteolytic product in its viral membrane-bound state. We found that some mutations, such as G123E and H219Q, involve direct interaction with cleavage site residues to influence their local environment, while certain mutations in the matrix domain lead to the enrichment of lipids important for Gag targeting and assembly. Collectively, our results reveal why non-cleavage site mutations have far-reaching implications outside of Gag proteolysis, with important consequences for drugging Gag maturation intermediates and tackling protease inhibitor resistance.
中文翻译:

综合建模和模拟显示 Gag 非切割位点突变对 HIV-1 病毒适应性的影响
逆转录病毒的高突变率是耐药性的主要原因之一。在人类免疫缺陷病毒 1 型 (HIV-1) 中,其蛋白酶和蛋白酶底物(组特异性抗原 (Gag) 多蛋白)的协同突变共同作用,赋予对蛋白酶抑制剂的耐药性,并补偿影响病毒适应性的突变。一些 Gag 突变可以恢复 Gag 蛋白酶结合,但大多数 Gag 蛋白酶相关突变发生在 Gag 切割位点之外。为了研究其分子基础,我们现在报告多尺度建模方法,以在切割位点之外发生的临床相关突变的背景下研究各种顺序切割的 Gag 产物,包括模拟其病毒膜结合的最大 Gag 蛋白水解产物状态。我们发现一些突变,例如 G123E 和 H219Q,涉及与裂解位点残基的直接相互作用以影响其局部环境,而基质结构域中的某些突变导致对 Gag 靶向和组装重要的脂质富集。总的来说,我们的结果揭示了为什么非切割位点突变在 Gag 蛋白水解之外具有深远的影响,对药物 Gag 成熟中间体和解决蛋白酶抑制剂耐药性具有重要影响。
更新日期:2020-12-23
中文翻译:

综合建模和模拟显示 Gag 非切割位点突变对 HIV-1 病毒适应性的影响
逆转录病毒的高突变率是耐药性的主要原因之一。在人类免疫缺陷病毒 1 型 (HIV-1) 中,其蛋白酶和蛋白酶底物(组特异性抗原 (Gag) 多蛋白)的协同突变共同作用,赋予对蛋白酶抑制剂的耐药性,并补偿影响病毒适应性的突变。一些 Gag 突变可以恢复 Gag 蛋白酶结合,但大多数 Gag 蛋白酶相关突变发生在 Gag 切割位点之外。为了研究其分子基础,我们现在报告多尺度建模方法,以在切割位点之外发生的临床相关突变的背景下研究各种顺序切割的 Gag 产物,包括模拟其病毒膜结合的最大 Gag 蛋白水解产物状态。我们发现一些突变,例如 G123E 和 H219Q,涉及与裂解位点残基的直接相互作用以影响其局部环境,而基质结构域中的某些突变导致对 Gag 靶向和组装重要的脂质富集。总的来说,我们的结果揭示了为什么非切割位点突变在 Gag 蛋白水解之外具有深远的影响,对药物 Gag 成熟中间体和解决蛋白酶抑制剂耐药性具有重要影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号