Cell ( IF 45.5 ) Pub Date : 2020-12-23 , DOI: 10.1016/j.cell.2020.11.038 Rie Nygaard 1 , Jia Yu 2 , Jonathan Kim 1 , Daniel R Ross 1 , Giacomo Parisi 1 , Oliver B Clarke 3 , David M Virshup 4 , Filippo Mancia 1
|
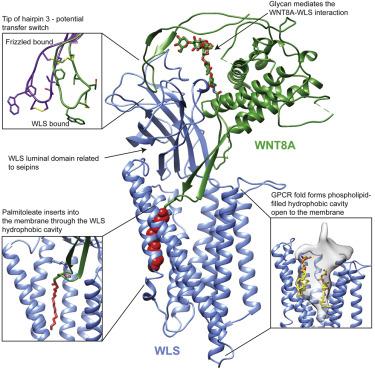
Wnts are evolutionarily conserved ligands that signal at short range to regulate morphogenesis, cell fate, and stem cell renewal. The first and essential steps in Wnt secretion are their O-palmitoleation and subsequent loading onto the dedicated transporter Wntless/evenness interrupted (WLS/Evi). We report the 3.2 Å resolution cryogenic electron microscopy (cryo-EM) structure of palmitoleated human WNT8A in complex with WLS. This is accompanied by biochemical experiments to probe the physiological implications of the observed association. The WLS membrane domain has close structural homology to G protein-coupled receptors (GPCRs). A Wnt hairpin inserts into a conserved hydrophobic cavity in the GPCR-like domain, and the palmitoleate protrudes between two helices into the bilayer. A conformational switch of highly conserved residues on a separate Wnt hairpin might contribute to its transfer to receiving cells. This work provides molecular-level insights into a central mechanism in animal body plan development and stem cell biology.
中文翻译:

WLS/Evi 介导的 Wnt 转运和分泌的结构基础
Wnt 是进化上保守的配体,可在短距离内发出信号以调节形态发生、细胞命运和干细胞更新。 Wnt 分泌的首要步骤是 O-棕榈油化,然后加载到专用转运蛋白 Wntless/均匀性中断 (WLS/Evi) 上。我们报道了棕榈油化人 WNT8A 与 WLS 复合物的 3.2 Å 分辨率低温电子显微镜 (cryo-EM) 结构。同时进行生化实验,以探讨所观察到的关联的生理学意义。 WLS 膜结构域与 G 蛋白偶联受体 (GPCR) 具有密切的结构同源性。 Wnt 发夹插入 GPCR 样结构域中的保守疏水空腔,棕榈油酸酯在两个螺旋之间突出到双层中。单独的 Wnt 发夹上高度保守残基的构象转换可能有助于其转移到接受细胞。这项工作为动物身体计划发展和干细胞生物学的中心机制提供了分子水平的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号