当前位置:
X-MOL 学术
›
Isr. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective C(sp2)−C(sp3) Oxidative Bond Cleavage of 1-(1-Hydroxyalkyl) naphthalen-2-ols: First Synthesis of 1-Azido-halo-naphthalene-2(1H)-ones
Israel Journal of Chemistry ( IF 2.3 ) Pub Date : 2020-12-22 , DOI: 10.1002/ijch.202000082 Manoj Kumar Ghosh 1 , Barnali Roy 1 , Debayan Sarkar 1
Israel Journal of Chemistry ( IF 2.3 ) Pub Date : 2020-12-22 , DOI: 10.1002/ijch.202000082 Manoj Kumar Ghosh 1 , Barnali Roy 1 , Debayan Sarkar 1
Affiliation
|
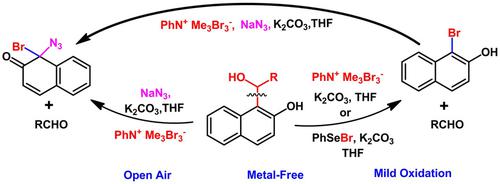
A new one-pot oxidative cleavage of C(sp2)−C(sp3) bond has been explored using two complementary protocols employing Phenylselenyl bromide (PhSeBr) as well as Phenyltrimethylammonium Tribromide (PTAB), with much greater efficiency and scope. The oxidative cleavage proceeds via a dearomatization step to afford potentially useful bromonaphthols along with corresponding aldehydes. In this course, we also described the first synthesis of synthetically important azido-halo naphthaleneones.
中文翻译:

1-(1-羟基烷基) naphthalen-2-ols 的区域选择性 C(sp2)-C(sp3) 氧化键裂解:1-叠氮基-卤代-萘-2(1H)-ones 的首次合成
使用苯基硒基溴 (PhSeBr) 和苯基三甲基三溴化铵 (PTAB) 的两种互补方案探索了 C(sp 2 )-C(sp 3 ) 键的新一锅氧化裂解,具有更高的效率和范围。氧化裂解通过脱芳构化步骤进行,以提供潜在有用的溴萘酚和相应的醛。在本课程中,我们还描述了合成重要的叠氮基卤代萘的首次合成。
更新日期:2020-12-22
中文翻译:

1-(1-羟基烷基) naphthalen-2-ols 的区域选择性 C(sp2)-C(sp3) 氧化键裂解:1-叠氮基-卤代-萘-2(1H)-ones 的首次合成
使用苯基硒基溴 (PhSeBr) 和苯基三甲基三溴化铵 (PTAB) 的两种互补方案探索了 C(sp 2 )-C(sp 3 ) 键的新一锅氧化裂解,具有更高的效率和范围。氧化裂解通过脱芳构化步骤进行,以提供潜在有用的溴萘酚和相应的醛。在本课程中,我们还描述了合成重要的叠氮基卤代萘的首次合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号