当前位置:
X-MOL 学术
›
Cryst. Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Bonding Interactions in Fluorinated Vinylogous Amides: A CF3‐Substituted Carbonyl‐β‐Aminoenone as a Case Study
Crystal Research and Technology ( IF 1.5 ) Pub Date : 2020-12-22 , DOI: 10.1002/crat.202000162 Edeimis Espitia Cogollo 1 , Eliana Jios 1 , Alejandra Hidalgo 1 , Sonia Elizabeth Ulic 1, 2 , Gustavo Alberto Echeverría 3 , Oscar Enrique Piro 3 , Jorge Luis Jios 4, 5
Crystal Research and Technology ( IF 1.5 ) Pub Date : 2020-12-22 , DOI: 10.1002/crat.202000162 Edeimis Espitia Cogollo 1 , Eliana Jios 1 , Alejandra Hidalgo 1 , Sonia Elizabeth Ulic 1, 2 , Gustavo Alberto Echeverría 3 , Oscar Enrique Piro 3 , Jorge Luis Jios 4, 5
Affiliation
|
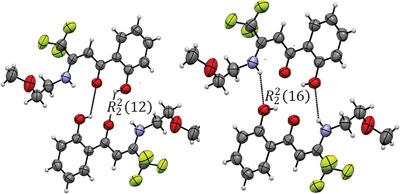
A new perfluoromethylated vinylogous amide, (Z)‐4,4,4‐trifluoro‐1‐(2‐hydroxyphenyl)‐3‐(2‐methoxyethylamino)‐2‐buten‐1‐one, is chosen as an example to investigate the bonding interactions in solid state. The Z, s‐cis form is the dominant conformation in both solution and solid state. Intramolecular hydrogen bonding determines this conformational preference in a nonpolar solvent (NMR spectra). Carbonyl and phenol groups are sensitive to the intra‐ and intermolecular contacts (vibrational spectra). The supramolecular assembly (X‐ray diffraction) is governed by NH⋯O and OH⋯O strong intermolecular hydrogen bonds giving rise to center‐symmetric and graph‐set motifs. The π‐stacking, F⋯H and F⋯F interactions are also discussed (Hirshfeld analysis).
中文翻译:

氟化乙烯基酰胺中的键相互作用:以CF3取代的羰基-β-氨基酮为例
以一种新的全氟甲基化乙烯基酰胺(Z)-4,4,4,4-三氟-1-(2-羟基苯基)-3-(2-甲氧基乙基氨基)-2-丁烯-1-酮为例来研究固态键合相互作用。Z,顺式是溶液和固态的主要构象。分子内氢键决定了在非极性溶剂中的构象偏好(NMR光谱)。羰基和酚基对分子内和分子间接触(振动光谱)敏感。超分子组装(X射线衍射)受NH⋯O和OH⋯O的强分子间氢键控制,从而产生中心对称 和 图形设置的图案。还讨论了π堆积,F⋯H和F⋯F相互作用(Hirshfeld分析)。
更新日期:2021-02-11
中文翻译:

氟化乙烯基酰胺中的键相互作用:以CF3取代的羰基-β-氨基酮为例
以一种新的全氟甲基化乙烯基酰胺(Z)-4,4,4,4-三氟-1-(2-羟基苯基)-3-(2-甲氧基乙基氨基)-2-丁烯-1-酮为例来研究固态键合相互作用。Z,顺式是溶液和固态的主要构象。分子内氢键决定了在非极性溶剂中的构象偏好(NMR光谱)。羰基和酚基对分子内和分子间接触(振动光谱)敏感。超分子组装(X射线衍射)受NH⋯O和OH⋯O的强分子间氢键控制,从而产生中心对称 和 图形设置的图案。还讨论了π堆积,F⋯H和F⋯F相互作用(Hirshfeld分析)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号