当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Crystal Structure, Biological Evaluation and in Silico Studies on Novel (E)‐1‐(substituted benzylidene)‐4‐(3‐isopropylphenyl)thiosemicarbazone Derivatives
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2021-01-14 , DOI: 10.1002/cbdv.202000804 Fan Qi 1 , Qianqian Qi 2 , Jirong Song 3 , Jie Huang 1
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2021-01-14 , DOI: 10.1002/cbdv.202000804 Fan Qi 1 , Qianqian Qi 2 , Jirong Song 3 , Jie Huang 1
Affiliation
|
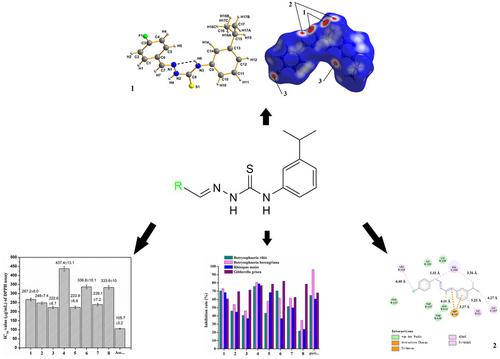
A series of (E)-1-(substituted benzylidene)-4-(3-isopropylphenyl)thiosemicarbazone derivatives were synthesized and characterized by FT-IR spectrum, elemental analysis, NMR spectrum, HRMS spectrum and X-ray single crystal diffraction technology. The crystal structures and packing of (E)-1-(4-fluorobenzylidene)-4-(3-isopropylphenyl)thiosemicarbazone and (E)-1-(3-fluorobenzylidene)-4-(3-isopropylphenyl)thiosemicarbazone were maintained through the intramolecular hydrogen bond (N3-H6···N1) and intermolecular hydrogen bonds (N2-H4···S1, C14-H14···F1 and C7-H7···S1). The results obtained by employing the DPPH free radicals scavenging assay indicated that (E)-1-(4-methoxylbenzylidene)-4-(3-isopropylphenyl)thiosemicarbazone had a more significant antioxidant activity compared with other compounds. The results measured by adopting the disc diffusion method elucidated that (E)-1-(4-trifluoromethylbenzylidene)-4-(3-isopropylphenyl)thiosemicarbazone possessed a more prominent antifungal activity than other compounds. Molecular docking showed that (E)-1-(4-chlorobenzylidene)-4-(3-isopropylphenyl)thiosemicarbazone had the highest affinity with receptor protein (1NMT). Moreover, the drug-likeness characteristic, physicochemical properties, pharmacokinetic profiles and bioactivity scores of all the compounds were predicted through in silico studies. The results illustrated that (E)-1-(4-fluorobenzylidene)-4-(3-isopropylphenyl)thiosemicarbazone had the drug-likeness characteristic and all the compounds were considered as moderately biological active molecules.
中文翻译:

新型 (E)-1-(取代亚苄基)-4-(3-异丙基苯基)缩氨基硫脲衍生物的合成、晶体结构、生物学评价和硅研究
合成了一系列(E)-1-(取代亚苄基)-4-(3-异丙基苯基)缩氨基硫脲衍生物,并通过FT-IR光谱、元素分析、NMR光谱、HRMS光谱和X射线单晶衍射技术进行了表征。(E)-1-(4-氟亚苄基)-4-(3-异丙基苯基)缩氨基硫脲和(E)-1-(3-氟亚苄基)-4-(3-异丙基苯基)缩氨基硫脲的晶体结构和堆积通过分子内氢键(N3-H6...N1)和分子间氢键(N2-H4...S1、C14-H14...F1 和 C7-H7...S1)。DPPH自由基清除实验结果表明,(E)-1-(4-甲氧基亚苄基)-4-(3-异丙基苯基)缩氨基硫脲比其他化合物具有更显着的抗氧化活性。采用圆盘扩散法测定的结果表明,(E)-1-(4-三氟甲基亚苄基)-4-(3-异丙基苯基)缩氨基硫脲比其他化合物具有更显着的抗真菌活性。分子对接显示(E)-1-(4-氯亚苄基)-4-(3-异丙基苯基)缩氨基硫脲与受体蛋白(1NMT)的亲和力最高。此外,通过计算机研究预测了所有化合物的药物相似性特征、理化特性、药代动力学特征和生物活性评分。结果表明(E)-1-(4-氟亚苄基)-4-(3-异丙基苯基)缩氨基硫脲具有类药性特征,所有化合物均被认为是中等生物活性分子。
更新日期:2021-01-14
中文翻译:

新型 (E)-1-(取代亚苄基)-4-(3-异丙基苯基)缩氨基硫脲衍生物的合成、晶体结构、生物学评价和硅研究
合成了一系列(E)-1-(取代亚苄基)-4-(3-异丙基苯基)缩氨基硫脲衍生物,并通过FT-IR光谱、元素分析、NMR光谱、HRMS光谱和X射线单晶衍射技术进行了表征。(E)-1-(4-氟亚苄基)-4-(3-异丙基苯基)缩氨基硫脲和(E)-1-(3-氟亚苄基)-4-(3-异丙基苯基)缩氨基硫脲的晶体结构和堆积通过分子内氢键(N3-H6...N1)和分子间氢键(N2-H4...S1、C14-H14...F1 和 C7-H7...S1)。DPPH自由基清除实验结果表明,(E)-1-(4-甲氧基亚苄基)-4-(3-异丙基苯基)缩氨基硫脲比其他化合物具有更显着的抗氧化活性。采用圆盘扩散法测定的结果表明,(E)-1-(4-三氟甲基亚苄基)-4-(3-异丙基苯基)缩氨基硫脲比其他化合物具有更显着的抗真菌活性。分子对接显示(E)-1-(4-氯亚苄基)-4-(3-异丙基苯基)缩氨基硫脲与受体蛋白(1NMT)的亲和力最高。此外,通过计算机研究预测了所有化合物的药物相似性特征、理化特性、药代动力学特征和生物活性评分。结果表明(E)-1-(4-氟亚苄基)-4-(3-异丙基苯基)缩氨基硫脲具有类药性特征,所有化合物均被认为是中等生物活性分子。










































 京公网安备 11010802027423号
京公网安备 11010802027423号