当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reduction of silver ions to form silver nanoparticles by redox-active organic molecules: coupled impact of the redox state and environmental factors
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-12-9 , DOI: 10.1039/d0en00820f Feng Dong 1, 2, 3, 4, 5 , Chao Wu 5, 6, 7, 8, 9 , Ai-Jun Miao 5, 6, 7, 8, 9 , Ke Pan 1, 2, 3, 4, 5
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-12-9 , DOI: 10.1039/d0en00820f Feng Dong 1, 2, 3, 4, 5 , Chao Wu 5, 6, 7, 8, 9 , Ai-Jun Miao 5, 6, 7, 8, 9 , Ke Pan 1, 2, 3, 4, 5
Affiliation
|
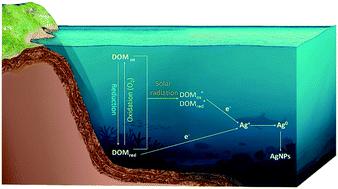
The release of silver ions (Ag+) is an important process in determining the environmental fate and toxic effects of silver nanoparticles (AgNPs), but the released Ag+ can be reduced to form new AgNPs in environments. Here the reduction of Ag+ by redox-active organic molecules to form AgNPs under different lighting and oxygen conditions was investigated using anthraquinonoe-2,6-disulphonate (AQDS) and nicotinamide adenine dinucleotide (NAD+) as representatives. In anaerobic environments, oxidized AQDS (AQDSox) and NAD+ failed to reduce Ag+ to form AgNPs, whereas reduced AQDS (AQDSred) and NAD+ (NADH) produced a concentration-dependent AgNP production pattern, underlining a key role of the redox state in Ag+ reduction by organics. The presence of O2 diminished the reducing capacity of NADH and AQDSred, probably due to their oxidation by O2. However, under UV light, the AgNP formation rate was dramatically enhanced in AQDS, independent of O2. Our results revealed that the Ag+ photoreduction was mainly attributed to the photoexcited AQDS, not the radical intermediates. For the complex organic humic acid (HA), and consistent with the phenomena in AQDS, the reduced HA (HAred) reduced Ag+ at a much faster rate than the native HA (HAox) in anaerobic and dark environments, and the amounts of AgNPs produced by HAred decreased in oxic environments. UV illumination enabled significant Ag+ reduction in both HAox and HAred, and O2 promoted the AgNP formation. Our study systematically revealed different Ag+-reduction pathways to form AgNPs, with an organic's redox state and environmental factors having a combined effect.
中文翻译:

氧化还原活性有机分子还原银离子形成银纳米粒子:氧化还原状态和环境因素的耦合影响
银离子(Ag +)的释放是确定银纳米颗粒(AgNPs)的环境命运和毒性影响的重要过程,但是释放的Ag +可以被还原以在环境中形成新的AgNPs。在这里,以蒽醌-2,6-二磺酸盐(AQDS)和烟酰胺腺嘌呤二核苷酸(NAD +)为代表,研究了氧化还原活性有机分子在不同的光照和氧气条件下还原Ag +形成AgNPs的方法。在厌氧环境中,氧化的AQDS(AQDS ox)和NAD +无法还原Ag +形成AgNPs,而还原的AQDS(AQDS red)和NAD +(NADH)产生了浓度依赖性的AgNP生产模式,强调了氧化还原状态在有机物还原Ag +中的关键作用。O 2的存在降低了NADH和AQDS red的还原能力,这可能是由于它们被O 2氧化了。但是,在紫外线下,AQDS中的AgNP形成速率显着提高,而与O 2无关。我们的结果表明,Ag +的光还原主要归因于光激发的AQDS,而不是自由基中间体。对于复杂的有机腐殖酸(HA),并与AQDS中的现象一致,还原的HA(HA red)还原了Ag +在厌氧和黑暗环境中,其速度要比天然HA(HA ox)快得多,并且在有氧环境中,由HA red产生的AgNPs数量减少。紫外线照射可显着降低HA ox和HA red中的Ag +含量,而O 2促进AgNP的形成。我们的研究系统地揭示了形成AgNPs的不同的Ag +-还原途径,其中有机物的氧化还原状态和环境因素具有综合作用。
更新日期:2020-12-21
中文翻译:

氧化还原活性有机分子还原银离子形成银纳米粒子:氧化还原状态和环境因素的耦合影响
银离子(Ag +)的释放是确定银纳米颗粒(AgNPs)的环境命运和毒性影响的重要过程,但是释放的Ag +可以被还原以在环境中形成新的AgNPs。在这里,以蒽醌-2,6-二磺酸盐(AQDS)和烟酰胺腺嘌呤二核苷酸(NAD +)为代表,研究了氧化还原活性有机分子在不同的光照和氧气条件下还原Ag +形成AgNPs的方法。在厌氧环境中,氧化的AQDS(AQDS ox)和NAD +无法还原Ag +形成AgNPs,而还原的AQDS(AQDS red)和NAD +(NADH)产生了浓度依赖性的AgNP生产模式,强调了氧化还原状态在有机物还原Ag +中的关键作用。O 2的存在降低了NADH和AQDS red的还原能力,这可能是由于它们被O 2氧化了。但是,在紫外线下,AQDS中的AgNP形成速率显着提高,而与O 2无关。我们的结果表明,Ag +的光还原主要归因于光激发的AQDS,而不是自由基中间体。对于复杂的有机腐殖酸(HA),并与AQDS中的现象一致,还原的HA(HA red)还原了Ag +在厌氧和黑暗环境中,其速度要比天然HA(HA ox)快得多,并且在有氧环境中,由HA red产生的AgNPs数量减少。紫外线照射可显着降低HA ox和HA red中的Ag +含量,而O 2促进AgNP的形成。我们的研究系统地揭示了形成AgNPs的不同的Ag +-还原途径,其中有机物的氧化还原状态和环境因素具有综合作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号