当前位置:
X-MOL 学术
›
Immunology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanoparticle‐mediated targeting of autoantigen peptide to cross‐presenting liver sinusoidal endothelial cells protects from CD8 T‐cell‐driven autoimmune cholangitis
Immunology ( IF 4.9 ) Pub Date : 2020-12-21 , DOI: 10.1111/imm.13298 Antonella Carambia 1 , Cornelia Gottwick 1 , Dorothee Schwinge 1 , Stephanie Stein 1 , Reinaldo Digigow 2 , Muharrem Şeleci 2 , Disha Mungalpara 2 , Markus Heine 3 , Fenja A Schuran 1 , Carlotta Corban 3 , Ansgar W Lohse 1 , Christoph Schramm 1, 4 , Joerg Heeren 3 , Johannes Herkel 1
Immunology ( IF 4.9 ) Pub Date : 2020-12-21 , DOI: 10.1111/imm.13298 Antonella Carambia 1 , Cornelia Gottwick 1 , Dorothee Schwinge 1 , Stephanie Stein 1 , Reinaldo Digigow 2 , Muharrem Şeleci 2 , Disha Mungalpara 2 , Markus Heine 3 , Fenja A Schuran 1 , Carlotta Corban 3 , Ansgar W Lohse 1 , Christoph Schramm 1, 4 , Joerg Heeren 3 , Johannes Herkel 1
Affiliation
|
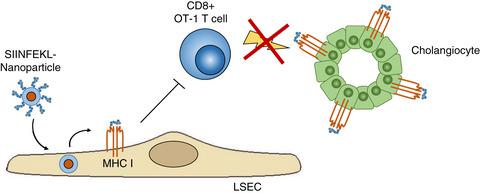
Autoimmune diseases are caused by adaptive immune responses to self‐antigens. The development of antigen‐specific therapies that suppress disease‐related, but not unrelated immune responses in general, is an important goal of biomedical research. We have previously shown that delivery of myelin peptides to liver sinusoidal endothelial cells (LSECs) using LSEC‐targeting nanoparticles provides effective protection from CD4 T‐cell‐driven autoimmune encephalomyelitis. Here, we investigated whether this methodology might also serve antigen‐specific treatment of a CD8 T‐cell‐driven autoimmune disease. As a model for CD8 T‐cell‐mediated autoimmunity, we used OT‐1 T‐cell‐driven cholangitis in K14‐OVAp mice expressing the cognate MHC I‐restricted SIINFEKL peptide in cholangiocytes. To study whether peptide delivery to LSECs could modulate cholangitis, SIINFEKL peptide‐conjugated nanoparticles were administered intravenously one day before transfer of OT‐1 T cells; five days after cell transfer, liver pathology and hepatic infiltrates were analysed. SIINFEKL peptide‐conjugated nanoparticles were rapidly taken up by LSECs in vivo, which effectively cross‐presented the delivered peptide on MHC I molecules. Intriguingly, K14‐OVAp mice receiving SIINFEKL‐loaded nanoparticles manifested significantly reduced liver damage compared with vehicle‐treated K14‐OVAp mice. Mechanistically, treatment with LSEC‐targeting SIINFEKL‐loaded nanoparticles significantly reduced the number of liver‐infiltrating OT‐1 T cells, which up‐regulated expression of the co‐inhibitory receptor PD‐1 and down‐regulated cytotoxic effector function and inflammatory cytokine production. These findings show that tolerogenic LSECs can effectively internalize circulating nanoparticles and cross‐present nanoparticle‐bound peptides on MHC I molecules. Therefore, nanoparticle‐mediated autoantigen peptide delivery to LSECs might serve the antigen‐specific treatment of CD8 T‐cell‐driven autoimmune disease.
中文翻译:

纳米颗粒介导的自身抗原肽靶向交叉呈递肝窦内皮细胞可预防 CD8 T 细胞驱动的自身免疫性胆管炎
自身免疫性疾病是由对自身抗原的适应性免疫反应引起的。开发抑制与疾病相关但通常不相关的免疫反应的抗原特异性疗法是生物医学研究的一个重要目标。我们之前已经表明,使用 LSEC 靶向纳米颗粒将髓鞘肽递送至肝窦内皮细胞 (LSEC) 可有效预防 CD4 T 细胞驱动的自身免疫性脑脊髓炎。在这里,我们研究了这种方法是否也可以用于 CD8 T 细胞驱动的自身免疫性疾病的抗原特异性治疗。作为 CD8 T 细胞介导的自身免疫模型,我们在 K14-OVAp 小鼠中使用 OT-1 T 细胞驱动的胆管炎,在胆管细胞中表达同源 MHC I 限制性 SIINFEKL 肽。为了研究将肽递送至 LSEC 是否可以调节胆管炎,在转移 OT-1 T 细胞前一天静脉注射 SIINFEKL 肽偶联纳米颗粒;细胞转移后五天,分析肝脏病理学和肝脏浸润。SIINFEKL 肽偶联纳米颗粒在体内被 LSEC 迅速吸收,有效地将递送的肽交叉呈递到 MHC I 分子上。有趣的是,与载体治疗的 K14-OVAp 小鼠相比,接受负载 SIINFEKL 纳米颗粒的 K14-OVAp 小鼠表现出显着减轻的肝损伤。从机制上讲,用 LSEC 靶向 SIINFEKL 纳米颗粒处理显着减少了肝脏浸润性 OT-1 T 细胞的数量,从而上调了共抑制受体 PD-1 的表达并下调了细胞毒性效应子功能和炎性细胞因子的产生. 这些发现表明,致耐受性 LSEC 可以有效地内化循环纳米颗粒,并在 MHC I 分子上交叉呈递纳米颗粒结合肽。因此,纳米颗粒介导的自身抗原肽递送至 LSEC 可能有助于 CD8 T 细胞驱动的自身免疫性疾病的抗原特异性治疗。
更新日期:2020-12-21
中文翻译:

纳米颗粒介导的自身抗原肽靶向交叉呈递肝窦内皮细胞可预防 CD8 T 细胞驱动的自身免疫性胆管炎
自身免疫性疾病是由对自身抗原的适应性免疫反应引起的。开发抑制与疾病相关但通常不相关的免疫反应的抗原特异性疗法是生物医学研究的一个重要目标。我们之前已经表明,使用 LSEC 靶向纳米颗粒将髓鞘肽递送至肝窦内皮细胞 (LSEC) 可有效预防 CD4 T 细胞驱动的自身免疫性脑脊髓炎。在这里,我们研究了这种方法是否也可以用于 CD8 T 细胞驱动的自身免疫性疾病的抗原特异性治疗。作为 CD8 T 细胞介导的自身免疫模型,我们在 K14-OVAp 小鼠中使用 OT-1 T 细胞驱动的胆管炎,在胆管细胞中表达同源 MHC I 限制性 SIINFEKL 肽。为了研究将肽递送至 LSEC 是否可以调节胆管炎,在转移 OT-1 T 细胞前一天静脉注射 SIINFEKL 肽偶联纳米颗粒;细胞转移后五天,分析肝脏病理学和肝脏浸润。SIINFEKL 肽偶联纳米颗粒在体内被 LSEC 迅速吸收,有效地将递送的肽交叉呈递到 MHC I 分子上。有趣的是,与载体治疗的 K14-OVAp 小鼠相比,接受负载 SIINFEKL 纳米颗粒的 K14-OVAp 小鼠表现出显着减轻的肝损伤。从机制上讲,用 LSEC 靶向 SIINFEKL 纳米颗粒处理显着减少了肝脏浸润性 OT-1 T 细胞的数量,从而上调了共抑制受体 PD-1 的表达并下调了细胞毒性效应子功能和炎性细胞因子的产生. 这些发现表明,致耐受性 LSEC 可以有效地内化循环纳米颗粒,并在 MHC I 分子上交叉呈递纳米颗粒结合肽。因此,纳米颗粒介导的自身抗原肽递送至 LSEC 可能有助于 CD8 T 细胞驱动的自身免疫性疾病的抗原特异性治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号