当前位置:
X-MOL 学术
›
Nano Today
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A versatile strategy for improving phototherapeutic efficacy on deep-sited tumor by tissue optical clearing technique
Nano Today ( IF 13.2 ) Pub Date : 2020-12-20 , DOI: 10.1016/j.nantod.2020.101058 Hao Zhao , Jiabao Xu , Jiangshan Wan , Wenjing Huang , Yanbing Zhao , Xiangliang Yang
Nano Today ( IF 13.2 ) Pub Date : 2020-12-20 , DOI: 10.1016/j.nantod.2020.101058 Hao Zhao , Jiabao Xu , Jiangshan Wan , Wenjing Huang , Yanbing Zhao , Xiangliang Yang
|
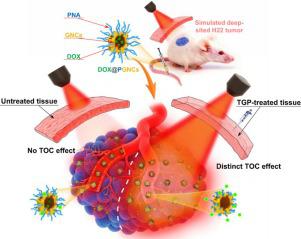
Phototherapies are almost entirely ineffective against deep-sited tumors, due to the poor tissue-penetration ability of light, including ultraviolet light, visible light and infrared light. Although tissue optical clearing (TOC) technique, based on refractive index matching between scatterers and media, has been used to improve optical imaging quality, it is the first time that TOC technique was employed to realize efficient phototherapeutic effect on deep-sited tumor in the present work. A hybrid TOC agent consisting of glycerol, polyethylene glycol 400 and butylbenisothiazolene (TGP), were optimized for achieving the synergistic antitumor efficacy of photothermal therapy (PTT) and chemotherapy on a simulated deep-sited tumor model (with the coverage of pigskin). Temperature-sensitive gold nanocages (PGNCs) was used to load doxorubicin (DOX@PGNCs) as a nano-platform model of PTT and chemotherapy, for a proof of concept to prove the TOC effect of TGP on the phototherapeutic efficacy against deep-sited tumor. As the pigskin was treated by TGP for 30 min, the ΔT of DOX@PGNCs increased to 12.1 °C under NIR irradiation, while it only increased to 2.0 °C with the coverage of untreated-pigskin. Moreover, PTT-triggered release of DOX increased from 6.3% with the coverage of untreated pigskin up to 22.8% with the coverage of TGP-treated pigskin under three times of NIR irradiation, indicating enhanced photothermal conversion efficiency. Meanwhile, the TOC effect of TGP enhanced cellular uptakes of DOX@PGNCs and boosted delivery efficiency of DOX@PGNCs into the tumor by photothermal-induced hydrophilicity–hydrophobicity transition. Owing to the improvement on the tissue-penetration depth of NIR light, DOX@PGNCs accomplished a robustly synergistic antitumor efficacy of PTT-chemotherapy on a deep-sited H22 tumor model. It is promising to be developed as a versatile strategy for improving PTT efficacy on deep-sited tumors and showed great potential in the improvement of clinical application of various phototherapies.
中文翻译:

通过组织光透明技术提高深部肿瘤光疗效果的通用策略
由于光(包括紫外线、可见光和红外线)的组织穿透能力差,光疗法对深部肿瘤几乎完全无效。虽然基于散射体和介质之间折射率匹配的组织光学清除(TOC)技术已被用于提高光学成像质量,但利用TOC技术对深部肿瘤实现高效光疗效果尚属首次。目前的工作。优化了由甘油、聚乙二醇400和丁基苯异噻唑啉(TGP)组成的混合TOC剂,以在模拟深部肿瘤模型(猪皮覆盖)上实现光热疗法(PTT)和化疗的协同抗肿瘤功效。使用温敏金纳米笼(PGNCs)负载阿霉素(DOX@PGNCs)作为PTT和化疗的纳米平台模型,以概念验证TGP对深部肿瘤光疗疗效的TOC效应。当猪皮经 TGP 处理 30 分钟后,在近红外辐射下 DOX@PGNCs 的 ΔT 增加至 12.1 °C,而未处理猪皮的覆盖率仅增加至 2.0 °C。此外,在三倍近红外照射下,PTT触发的DOX释放量从未处理猪皮的6.3%增加到经过TGP处理的猪皮的22.8%,表明光热转换效率增强。同时,TGP的TOC效应增强了DOX@PGNCs的细胞摄取,并通过光热诱导的亲水性-疏水性转变提高了DOX@PGNCs进入肿瘤的递送效率。由于近红外光组织穿透深度的改善,DOX@PGNCs 在深部 H22 肿瘤模型上实现了 PTT 化疗的强大协同抗肿瘤功效。它有望被开发为一种提高深部肿瘤 PTT 疗效的通用策略,并在改善各种光疗法的临床应用方面显示出巨大潜力。
更新日期:2020-12-20
中文翻译:

通过组织光透明技术提高深部肿瘤光疗效果的通用策略
由于光(包括紫外线、可见光和红外线)的组织穿透能力差,光疗法对深部肿瘤几乎完全无效。虽然基于散射体和介质之间折射率匹配的组织光学清除(TOC)技术已被用于提高光学成像质量,但利用TOC技术对深部肿瘤实现高效光疗效果尚属首次。目前的工作。优化了由甘油、聚乙二醇400和丁基苯异噻唑啉(TGP)组成的混合TOC剂,以在模拟深部肿瘤模型(猪皮覆盖)上实现光热疗法(PTT)和化疗的协同抗肿瘤功效。使用温敏金纳米笼(PGNCs)负载阿霉素(DOX@PGNCs)作为PTT和化疗的纳米平台模型,以概念验证TGP对深部肿瘤光疗疗效的TOC效应。当猪皮经 TGP 处理 30 分钟后,在近红外辐射下 DOX@PGNCs 的 ΔT 增加至 12.1 °C,而未处理猪皮的覆盖率仅增加至 2.0 °C。此外,在三倍近红外照射下,PTT触发的DOX释放量从未处理猪皮的6.3%增加到经过TGP处理的猪皮的22.8%,表明光热转换效率增强。同时,TGP的TOC效应增强了DOX@PGNCs的细胞摄取,并通过光热诱导的亲水性-疏水性转变提高了DOX@PGNCs进入肿瘤的递送效率。由于近红外光组织穿透深度的改善,DOX@PGNCs 在深部 H22 肿瘤模型上实现了 PTT 化疗的强大协同抗肿瘤功效。它有望被开发为一种提高深部肿瘤 PTT 疗效的通用策略,并在改善各种光疗法的临床应用方面显示出巨大潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号