Journal of Materials Research and Technology ( IF 6.4 ) Pub Date : 2020-12-19 , DOI: 10.1016/j.jmrt.2020.12.038 S.V. Ramos , P. Cisquini , R.C. Nascimento Jr. , A.R. Franco Jr. , E.A. Vieira
|
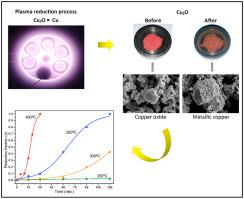
Non-thermal hydrogen plasma is a novel reducing medium that can yield H2 derived species capable of improving the reduction efficiency of metallic oxides when compared to a conventional reduction by hydrogen gas. To analyze the morphological changes of the samples and evaluate the impact of the plasma hydrogen species on the kinetic process, this work performed Cu2O powder reduction by non-thermal hydrogen plasma and compared it to hydrogen gas reduction at the same experimental conditions. The experiments were conducted in a pulsed direct current glow discharge reactor at pressure of 533 Pa, varying temperatures between 250°C and 400°C and reduction time intervals from 5 to 120 min. The results show that hydrogen plasma reduction is a faster process, reaching a higher degree of metallization of the Cu2O powder than hydrogen gas process in any reduction condition. Hydrogen plasma promotes a higher sintering degree of the newly formed porous copper particulates than hydrogen gas processing. The kinetic assessment of experimental data shows that plasma process kinetics are best-fitted to Prout-Tompkins’ nucleation model at temperature range from 300°C to 400°C, and to Jander's diffusion model at 250°C. On the other hand, gas process kinetics are best adjusted to Prout-Tompkins’ and JMAEK's models at 400°C and 350°C, respectively. The calculated apparent activation energy of plasma reduction is 19.06 kJ/mol at temperature range of 300°C – 400°C; whilst for gas reduction is 73.79 kJ/mol at temperature range of 350°C–400°C.
中文翻译:

非热氢等离子体还原Cu 2 O粉末的形貌变化和动力学评估
非热氢等离子体是一种新颖的还原介质,与常规的氢气还原相比,它可以产生H 2衍生的物种,该物种能够提高金属氧化物的还原效率。为了分析样品的形态变化并评估等离子体氢物种对动力学过程的影响,这项工作进行了Cu 2通过非热氢等离子体还原O粉末,并将其与相同实验条件下的氢气还原进行比较。实验在脉冲直流辉光放电反应器中进行,压力为533 Pa,温度在250°C至400°C之间变化,还原时间间隔为5至120分钟。结果表明,氢等离子体还原是一个更快的过程,达到了更高的Cu 2金属化程度O粉在任何还原条件下都比氢气处理。与氢气处理相比,氢等离子体促进新形成的多孔铜微粒的烧结度更高。对实验数据的动力学评估表明,等离子体工艺动力学最适合于温度范围为300°C至400°C的Prout-Tompkins形核模型,以及250°C的Jander扩散模型。另一方面,最好分别在400°C和350°C下根据Prout-Tompkins和JMAEK的模型调整气体处理动力学。在300°C – 400°C的温度范围内,等离子体还原的表观活化能为19.06 kJ / mol;在350°C–400°C的温度范围内,气体还原的速度为73.79 kJ / mol。



























 京公网安备 11010802027423号
京公网安备 11010802027423号