Journal of Chromatography B ( IF 2.8 ) Pub Date : 2020-12-19 , DOI: 10.1016/j.jchromb.2020.122508 Seong-Wook Seo , Ji-Min Kim , Dong-Gyun Han , Dongho Geum , Hwayoung Yun , In-Soo Yoon
|
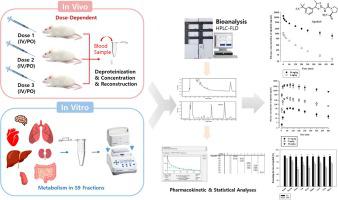
Alpelisib, a novel phosphatidylinositol 3-kinase inhibitor, is an oral anticancer agent approved for the treatment of advanced or metastatic breast cancer. In this study, a sensitive bioanalytical method using high-performance liquid chromatography combined with a fluorescence detector (HPLC-FLD) was developed for the determination of alpelisib in rat plasma. This newly developed method was validated in terms of linearity (1–1,000 ng/mL), precision, accuracy, recovery, matrix effect, and stability according to the US Food and Drug Administration guideline and these parameters were within the acceptable limits. Alpelisib tended to be stable in plasma, urine, simulated intestinal fluid, and buffer with pH > 4.0 for 24 h, but in the pH 1.2 buffer and simulated gastric fluid for up to 4 h only. A study involving intravenous administration of alpelisib in rats showed that the dose-normalized area under the plasma concentration versus time curve (AUC) of alpelisib changed significantly as the dose increased from 1 to 10 mg/kg. Similarly, an oral rat study indicated that the dose-normalized AUC and the fraction of dose that remained in the gastrointestinal (GI) tract changed significantly as the dose increased from 0.5 to 10 mg/kg. These nonlinear (dose-dependent) pharmacokinetics of intravenous and oral alpelisib could be attributed to the saturation of ubiquitous metabolism among most tissues and/or GI absorption processes. To the best of our knowledge, this is the first study to investigate the in vivo nonlinear pharmacokinetics of alpelisib and its possible mechanisms, together with a new HPLC-FLD method to determine alpelisib in biological matrices.
中文翻译:

灵敏的HPLC-FLD方法定量测定大鼠血浆中新型磷脂酰肌醇3激酶抑制剂alpelisib的体内和体外药物代谢和药代动力学评估
Alpelisib是一种新型磷脂酰肌醇3激酶抑制剂,是一种口服抗癌药,已被批准用于治疗晚期或转移性乳腺癌。在这项研究中,开发了一种使用高效液相色谱结合荧光检测器(HPLC-FLD)的灵敏生物分析方法,用于测定大鼠血浆中的alpelisib。根据美国食品和药物管理局的指导原则,这种新开发的方法在线性(1-1,000 ng / mL),精密度,准确性,回收率,基质效应和稳定性方面得到了验证,这些参数均在可接受的范围内。Alpelisib倾向于在血浆,尿液,模拟肠液和pH> 4.0的缓冲液中稳定24小时,但在pH 1.2缓冲液和模拟胃液中仅稳定4小时。一项涉及在大鼠中静脉内施用alpelisib的研究表明,随着剂量从1毫克/千克增加到10毫克/千克,alpelisib的血浆浓度对时间曲线下的剂量标准化面积发生了显着变化。同样,一项口服大鼠研究表明,随着剂量从0.5毫克/千克增加到10毫克/千克,归一化剂量的AUC和保留在胃肠道(GI)中的剂量比例发生了显着变化。静脉和口服alpelisib的这些非线性(剂量依赖性)药代动力学可以归因于大多数组织和/或GI吸收过程中普遍存在的新陈代谢的饱和。据我们所知,这是第一个研究alpelisib体内非线性药代动力学及其可能机制的研究,











































 京公网安备 11010802027423号
京公网安备 11010802027423号