当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Arylation of enelactams using TIPSOTf: reaction scope and mechanistic insight
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-12-7 , DOI: 10.1039/d0qo01396j Tomasz J. Idzik 1, 2, 2, 3, 4 , Zofia M. Myk 1, 2, 2, 3, 4 , Łukasz Struk 1, 2, 2, 3, 4 , Magdalena Perużyńska 5, 6, 7, 8 , Gabriela Maciejewska 8, 9, 10, 11 , Marek Droździk 5, 6, 7, 8 , Jacek G. Sośnicki 1, 2, 2, 3, 4
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-12-7 , DOI: 10.1039/d0qo01396j Tomasz J. Idzik 1, 2, 2, 3, 4 , Zofia M. Myk 1, 2, 2, 3, 4 , Łukasz Struk 1, 2, 2, 3, 4 , Magdalena Perużyńska 5, 6, 7, 8 , Gabriela Maciejewska 8, 9, 10, 11 , Marek Droździk 5, 6, 7, 8 , Jacek G. Sośnicki 1, 2, 2, 3, 4
Affiliation
|
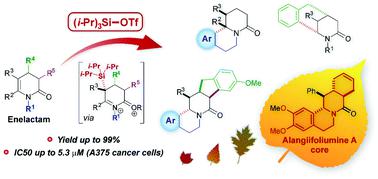
A novel method for the inter- and intramolecular arylation of enelactams (3,4-dihydropyridin-2-ones), up to 99% yield, triggered by triisopropylsilyltrifluoromethanesulfonate (TIPSOTf) is proposed. It offers high synthetic usefulness, especially for the synthesis of polycyclic systems obtained from conformationally rigid δ-enelactams, for which the use of the conventional method, involving cyclization by treatment with TfOH, failed. Multinuclear (1H, 13C, 19F and 29Si) NMR spectroscopy applied for the reaction monitoring as well as DOSY NMR experiment permitted identification of the intermediates and proposition of the reaction mechanism. In the process of checking the scope of the method application, condensed and bridged polycyclic piperidin-2-ones, including a derivative of alangiifoliumine A, were obtained. The inhibition of malignant melanoma A375 cell proliferation by some benzoquinolizidine derivatives (up to IC50 = 5.3 ± 0.4 μM) was evidenced.
中文翻译:

使用TIPSOTf烯内酰胺的丙烯酸化:反应范围和机理见解
提出了一种新的烯内酰胺(3,4-二氢吡啶-2-酮)分子间和分子内芳基化的方法,该方法可通过三异丙基甲硅烷基三氟甲磺酸盐(TIPSOTf)引发,产率高达99%。它提供了很高的合成实用性,特别是用于合成从构象刚性的δ-烯内酰胺获得的多环体系,为此,使用通过TfOH处理进行环化的常规方法失败了。多核(1 H,13 C,19 F和29Si)NMR光谱用于反应监测以及DOSY NMR实验,可以鉴定中间体和提出反应机理。在检查方法应用范围的过程中,获得了缩合和桥接的多环哌啶-2-酮,包括阿兰古西林A的衍生物。证实了某些苯并喹quin嗪衍生物(高达IC 50 = 5.3±0.4μM)对恶性黑色素瘤A375细胞增殖的抑制作用。
更新日期:2020-12-18
中文翻译:

使用TIPSOTf烯内酰胺的丙烯酸化:反应范围和机理见解
提出了一种新的烯内酰胺(3,4-二氢吡啶-2-酮)分子间和分子内芳基化的方法,该方法可通过三异丙基甲硅烷基三氟甲磺酸盐(TIPSOTf)引发,产率高达99%。它提供了很高的合成实用性,特别是用于合成从构象刚性的δ-烯内酰胺获得的多环体系,为此,使用通过TfOH处理进行环化的常规方法失败了。多核(1 H,13 C,19 F和29Si)NMR光谱用于反应监测以及DOSY NMR实验,可以鉴定中间体和提出反应机理。在检查方法应用范围的过程中,获得了缩合和桥接的多环哌啶-2-酮,包括阿兰古西林A的衍生物。证实了某些苯并喹quin嗪衍生物(高达IC 50 = 5.3±0.4μM)对恶性黑色素瘤A375细胞增殖的抑制作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号