当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rebuilding Ring-Type Assembly of Peroxiredoxin by Chemical Modification
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-12-17 , DOI: 10.1021/acs.bioconjchem.0c00587 Tomoki Himiyama 1, 2 , Yuko Tsuchiya 3 , Yasushige Yonezawa 4 , Tsutomu Nakamura 1, 2
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-12-17 , DOI: 10.1021/acs.bioconjchem.0c00587 Tomoki Himiyama 1, 2 , Yuko Tsuchiya 3 , Yasushige Yonezawa 4 , Tsutomu Nakamura 1, 2
Affiliation
|
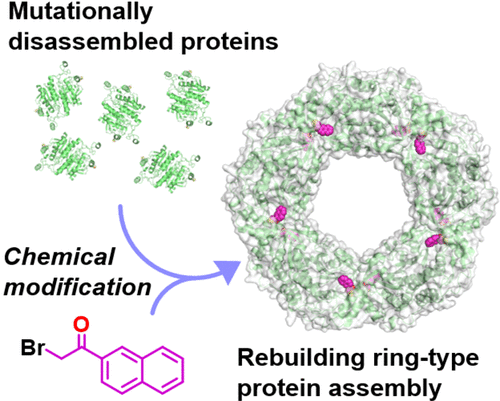
Direct control of the protein quaternary structure (QS) is challenging owing to the complexity of the protein structure. As a protein with a characteristic QS, peroxiredoxin from Aeropyrum pernix K1 (ApPrx) forms a decamer, wherein five dimers associate to form a ring. Here, we disrupted and reconstituted ApPrx QS via amino acid mutations and chemical modifications targeting hot spots for protein assembly. The decameric QS of an ApPrx* mutant, wherein all cysteine residues in wild-type ApPrx were mutated to serine, was destructed to dimers via an F80C mutation. The dimeric ApPrx*F80C mutant was then modified with a small molecule and successfully assembled as a decamer. Structural analysis confirmed that an artificially installed chemical moiety potentially facilitates suitable protein–protein interactions to rebuild a native structure. Rebuilding of dodecamer was also achieved through an additional amino acid mutation. This study describes a facile method to regulate the protein assembly state.
中文翻译:

通过化学修饰重建过氧化还原蛋白的环型组装
由于蛋白质结构的复杂性,直接控制蛋白质四级结构 (QS) 具有挑战性。作为具有特征性 QS 的蛋白质,来自Aeropyrum pernix 的过氧还蛋白K1 (ApPrx) 形成一个十聚体,其中五个二聚体结合形成一个环。在这里,我们通过针对蛋白质组装热点的氨基酸突变和化学修饰来破坏和重建 ApPrx QS。ApPrx* 突变体的十聚体 QS,其中野生型 ApPrx 中的所有半胱氨酸残基都突变为丝氨酸,通过 F80C 突变被破坏为二聚体。然后用小分子修饰二聚体 ApPrx*F80C 突变体并成功组装为十聚体。结构分析证实,人工安装的化学部分可能促进合适的蛋白质-蛋白质相互作用以重建天然结构。也通过额外的氨基酸突变实现了十二聚体的重建。本研究描述了一种调节蛋白质组装状态的简便方法。
更新日期:2021-01-20
中文翻译:

通过化学修饰重建过氧化还原蛋白的环型组装
由于蛋白质结构的复杂性,直接控制蛋白质四级结构 (QS) 具有挑战性。作为具有特征性 QS 的蛋白质,来自Aeropyrum pernix 的过氧还蛋白K1 (ApPrx) 形成一个十聚体,其中五个二聚体结合形成一个环。在这里,我们通过针对蛋白质组装热点的氨基酸突变和化学修饰来破坏和重建 ApPrx QS。ApPrx* 突变体的十聚体 QS,其中野生型 ApPrx 中的所有半胱氨酸残基都突变为丝氨酸,通过 F80C 突变被破坏为二聚体。然后用小分子修饰二聚体 ApPrx*F80C 突变体并成功组装为十聚体。结构分析证实,人工安装的化学部分可能促进合适的蛋白质-蛋白质相互作用以重建天然结构。也通过额外的氨基酸突变实现了十二聚体的重建。本研究描述了一种调节蛋白质组装状态的简便方法。









































 京公网安备 11010802027423号
京公网安备 11010802027423号