当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
l-Threonine Transaldolase Activity Is Enabled by a Persistent Catalytic Intermediate
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-12-18 , DOI: 10.1021/acschembio.0c00753 Prasanth Kumar 1 , Anthony Meza 2 , Jonathan M Ellis 1 , Grace A Carlson 1 , Craig A Bingman 2 , Andrew R Buller 1, 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-12-18 , DOI: 10.1021/acschembio.0c00753 Prasanth Kumar 1 , Anthony Meza 2 , Jonathan M Ellis 1 , Grace A Carlson 1 , Craig A Bingman 2 , Andrew R Buller 1, 2
Affiliation
|
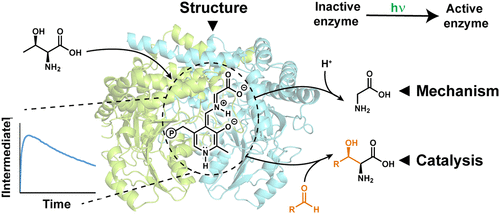
l-Threonine transaldolases (lTTAs) are a poorly characterized class of pyridoxal-5′-phosphate (PLP) dependent enzymes responsible for the biosynthesis of diverse β-hydroxy amino acids. Here, we study the catalytic mechanism of ObiH, an lTTA essential for biosynthesis of the β-lactone natural product obafluorin. Heterologously expressed ObiH purifies as a mixture of chemical states including a catalytically inactive form of the PLP cofactor. Photoexcitation of ObiH promotes the conversion of the inactive state of the enzyme to the active form. UV–vis spectroscopic analysis reveals that ObiH catalyzes the retro-aldol cleavage of l-threonine to form a remarkably persistent glycyl quinonoid intermediate, with a half-life of ∼3 h. Protonation of this intermediate is kinetically disfavored, enabling on-cycle reactivity with aldehydes to form β-hydroxy amino acids. We demonstrate the synthetic potential of ObiH via the single step synthesis of (2S,3R)-β-hydroxyleucine. To further understand the structural features underpinning this desirable reactivity, we determined the crystal structure of ObiH bound to PLP as the Schiff’s base at 1.66 Å resolution. This high-resolution model revealed a unique active site configuration wherein the evolutionarily conserved Asp that traditionally H-bonds to the cofactor is swapped for a neighboring Glu. Molecular dynamics simulations combined with mutagenesis studies indicate that a structural rearrangement is associated with l-threonine entry into the catalytic cycle. Together, these data explain the basis for the unique reactivity of lTTA enzymes and provide a foundation for future engineering and mechanistic analysis.
中文翻译:

l-苏氨酸转醛酶活性由持久的催化中间体启用
l-苏氨酸转醛缩酶 ( l TTA) 是一类缺乏特征的 5'-磷酸吡哆醛 (PLP) 依赖性酶,负责生物合成多种 β-羟基氨基酸。在这里,我们研究了 ObiH 的催化机制,ObiH 是一种对 β-内酯天然产物 obafluorin 的生物合成必不可少的l TTA。异源表达的 ObiH 纯化为化学状态的混合物,包括 PLP 辅因子的无催化活性形式。ObiH 的光激发促进酶的非活性状态向活性形式的转化。紫外-可见光谱分析表明,ObiH 催化l的逆醛醇裂解-苏氨酸形成显着持久的甘氨酰醌类中间体,半衰期约为 3 小时。这种中间体的质子化在动力学上是不利的,从而能够与醛进行循环反应以形成β-羟基氨基酸。我们通过 (2 S ,3 R ) 的单步合成证明了 ObiH 的合成潜力)-β-羟基亮氨酸。为了进一步了解支撑这种理想反应性的结构特征,我们以 1.66 Å 的分辨率确定了与 PLP 结合的 ObiH 的晶体结构作为席夫碱。这种高分辨率模型揭示了一种独特的活性位点配置,其中传统上与辅因子 H 键合的进化上保守的 Asp 被替换为相邻的 Glu。分子动力学模拟与诱变研究相结合,表明结构重排与l-苏氨酸进入催化循环有关。这些数据共同解释了l TTA 酶独特反应性的基础,并为未来的工程和机械分析奠定了基础。
更新日期:2021-01-15
中文翻译:

l-苏氨酸转醛酶活性由持久的催化中间体启用
l-苏氨酸转醛缩酶 ( l TTA) 是一类缺乏特征的 5'-磷酸吡哆醛 (PLP) 依赖性酶,负责生物合成多种 β-羟基氨基酸。在这里,我们研究了 ObiH 的催化机制,ObiH 是一种对 β-内酯天然产物 obafluorin 的生物合成必不可少的l TTA。异源表达的 ObiH 纯化为化学状态的混合物,包括 PLP 辅因子的无催化活性形式。ObiH 的光激发促进酶的非活性状态向活性形式的转化。紫外-可见光谱分析表明,ObiH 催化l的逆醛醇裂解-苏氨酸形成显着持久的甘氨酰醌类中间体,半衰期约为 3 小时。这种中间体的质子化在动力学上是不利的,从而能够与醛进行循环反应以形成β-羟基氨基酸。我们通过 (2 S ,3 R ) 的单步合成证明了 ObiH 的合成潜力)-β-羟基亮氨酸。为了进一步了解支撑这种理想反应性的结构特征,我们以 1.66 Å 的分辨率确定了与 PLP 结合的 ObiH 的晶体结构作为席夫碱。这种高分辨率模型揭示了一种独特的活性位点配置,其中传统上与辅因子 H 键合的进化上保守的 Asp 被替换为相邻的 Glu。分子动力学模拟与诱变研究相结合,表明结构重排与l-苏氨酸进入催化循环有关。这些数据共同解释了l TTA 酶独特反应性的基础,并为未来的工程和机械分析奠定了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号