当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
De Novo Discovery of High-Affinity Peptide Binders for the SARS-CoV-2 Spike Protein
ACS Central Science ( IF 12.7 ) Pub Date : 2020-12-17 , DOI: 10.1021/acscentsci.0c01309 Sebastian Pomplun 1 , Muhammad Jbara 1 , Anthony J Quartararo 1 , Genwei Zhang 1 , Joseph S Brown 1 , Yen-Chun Lee 1 , Xiyun Ye 1 , Stephanie Hanna 1 , Bradley L Pentelute 1, 2, 3, 4
ACS Central Science ( IF 12.7 ) Pub Date : 2020-12-17 , DOI: 10.1021/acscentsci.0c01309 Sebastian Pomplun 1 , Muhammad Jbara 1 , Anthony J Quartararo 1 , Genwei Zhang 1 , Joseph S Brown 1 , Yen-Chun Lee 1 , Xiyun Ye 1 , Stephanie Hanna 1 , Bradley L Pentelute 1, 2, 3, 4
Affiliation
|
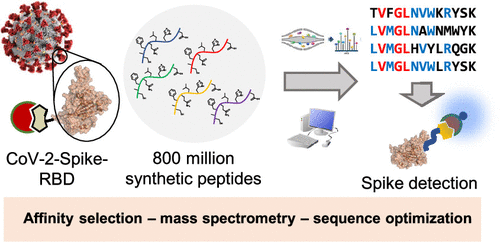
The β-coronavirus SARS-CoV-2 has caused a global pandemic. Affinity reagents targeting the SARS-CoV-2 spike protein are of interest for the development of therapeutics and diagnostics. We used affinity selection–mass spectrometry for the rapid discovery of synthetic high-affinity peptide binders for the receptor binding domain (RBD) of the SARS-CoV-2 spike protein. From library screening with 800 million synthetic peptides, we identified three sequences with nanomolar affinities (dissociation constants Kd = 80–970 nM) for RBD and selectivity over human serum proteins. Nanomolar RBD concentrations in a biological matrix could be detected using the biotinylated lead peptide in ELISA format. These peptides do not compete for ACE2 binding, and their site of interaction on the SARS-CoV-2-spike-RBD might be unrelated to the ACE2 binding site, making them potential orthogonal reagents for sandwich immunoassays. These findings serve as a starting point for the development of SARS-CoV-2 diagnostics or conjugates for virus-directed delivery of therapeutics.
中文翻译:

从头发现 SARS-CoV-2 刺突蛋白的高亲和力肽结合剂
β-冠状病毒 SARS-CoV-2 已引起全球大流行。针对 SARS-CoV-2 刺突蛋白的亲和试剂对于治疗和诊断的开发很有意义。我们使用亲和力选择-质谱法快速发现 SARS-CoV-2 刺突蛋白受体结合域 (RBD) 的合成高亲和力肽结合剂。通过对 8 亿条合成肽进行文库筛选,我们鉴定了 3 条 RBD 亲和力(解离常数K d = 80-970 nM)的序列,以及对人血清蛋白的选择性。可以使用 ELISA 格式的生物素化先导肽来检测生物基质中的纳摩尔 RBD 浓度。这些肽不会竞争 ACE2 结合,并且它们在 SARS-CoV-2-spike-RBD 上的相互作用位点可能与 ACE2 结合位点无关,这使它们成为夹心免疫测定的潜在正交试剂。这些发现可以作为开发 SARS-CoV-2 诊断方法或用于病毒定向治疗递送的结合物的起点。
更新日期:2021-01-27
中文翻译:

从头发现 SARS-CoV-2 刺突蛋白的高亲和力肽结合剂
β-冠状病毒 SARS-CoV-2 已引起全球大流行。针对 SARS-CoV-2 刺突蛋白的亲和试剂对于治疗和诊断的开发很有意义。我们使用亲和力选择-质谱法快速发现 SARS-CoV-2 刺突蛋白受体结合域 (RBD) 的合成高亲和力肽结合剂。通过对 8 亿条合成肽进行文库筛选,我们鉴定了 3 条 RBD 亲和力(解离常数K d = 80-970 nM)的序列,以及对人血清蛋白的选择性。可以使用 ELISA 格式的生物素化先导肽来检测生物基质中的纳摩尔 RBD 浓度。这些肽不会竞争 ACE2 结合,并且它们在 SARS-CoV-2-spike-RBD 上的相互作用位点可能与 ACE2 结合位点无关,这使它们成为夹心免疫测定的潜在正交试剂。这些发现可以作为开发 SARS-CoV-2 诊断方法或用于病毒定向治疗递送的结合物的起点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号