当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Microwave‐assisted synthesis and antimicrobial activity of novel spiro 1,3,4‐thiadiazolines from isatin derivatives
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-12-18 , DOI: 10.1002/jhet.4213 Daniel Pereira Costa 1 , Aleff Cruz Castro 1 , Girlyanderson Araújo Silva 1 , Claudio Gabriel Lima‐Junior 1 , Francisco Patricio Andrade Júnior 2 , Edeltrudes Oliveira Lima 3 , Boniek Gontijo Vaz 4 , Lidya Cardoso Silva 4
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-12-18 , DOI: 10.1002/jhet.4213 Daniel Pereira Costa 1 , Aleff Cruz Castro 1 , Girlyanderson Araújo Silva 1 , Claudio Gabriel Lima‐Junior 1 , Francisco Patricio Andrade Júnior 2 , Edeltrudes Oliveira Lima 3 , Boniek Gontijo Vaz 4 , Lidya Cardoso Silva 4
Affiliation
|
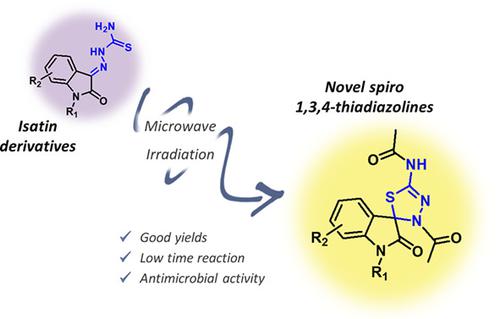
This work describes the synthesis of spiro 1,3,4‐thiadiazolines from isatin‐β‐thiosemicarbazone acetylation, using microwave irradiation as a source of heating the reaction medium. N‐substituted isatin derivatives were used as substrates to obtain thiosemicarbazones by adding thiosemicarbazide to the isatin ketone carbonyl. The final synthetic step was the reaction of thiosemicarbazones with acetic anhydride under microwave irradiation to get the spiro compounds. Reaction times ranged from 6 to 18 minutes resulting in yields of up to 90%. Biological assays have shown promising antibacterial and antifungal activity, especially spiro thiadiazolines derived from allylated isatins. All the proposed molecules proved to be potential drug candidates based on the results of the in silico investigation, with satisfactory drug‐likeness and drug‐score, respecting Lipinski's rule. The use of the microwave reactor was efficient for the synthesis of thiosemicarbazones and spiro compounds, resulting in a significant reduction in reaction times with conventional heating. Taking into account the threat of antimicrobial resistance, this work presents a series of bioactive molecules that are easily obtained via microwave reaction.
中文翻译:

靛红衍生物的新型螺线1,3,4-噻二唑啉的微波辅助合成和抗菌活性
这项工作描述了由Isatin-β-thiosemicarbazone乙酰化反应合成螺1,3,4-噻二唑啉,利用微波辐射作为加热反应介质的来源。ñ通过将thiosemicarbazide添加到isatin ketone羰基上,使用预先取代的isatin衍生物作为底物来获得thiosemicarbazones。最终的合成步骤是在微波辐射下硫代半氨基甲酮与乙酸酐的反应,得到螺环化合物。反应时间为6至18分钟,收率高达90%。生物学测定显示出有希望的抗菌和抗真菌活性,尤其是衍生自烯丙基化的靛红的螺噻二唑啉。根据计算机研究的结果,所有提出的分子均被证明是潜在的候选药物,并且符合Lipinski规则,具有令人满意的相似性和评分。微波反应器的使用对于合成硫半脲和螺环化合物非常有效,从而大大减少了常规加热的反应时间。考虑到抗药性的威胁,这项工作提出了一系列易于通过微波反应获得的生物活性分子。
更新日期:2020-12-18
中文翻译:

靛红衍生物的新型螺线1,3,4-噻二唑啉的微波辅助合成和抗菌活性
这项工作描述了由Isatin-β-thiosemicarbazone乙酰化反应合成螺1,3,4-噻二唑啉,利用微波辐射作为加热反应介质的来源。ñ通过将thiosemicarbazide添加到isatin ketone羰基上,使用预先取代的isatin衍生物作为底物来获得thiosemicarbazones。最终的合成步骤是在微波辐射下硫代半氨基甲酮与乙酸酐的反应,得到螺环化合物。反应时间为6至18分钟,收率高达90%。生物学测定显示出有希望的抗菌和抗真菌活性,尤其是衍生自烯丙基化的靛红的螺噻二唑啉。根据计算机研究的结果,所有提出的分子均被证明是潜在的候选药物,并且符合Lipinski规则,具有令人满意的相似性和评分。微波反应器的使用对于合成硫半脲和螺环化合物非常有效,从而大大减少了常规加热的反应时间。考虑到抗药性的威胁,这项工作提出了一系列易于通过微波反应获得的生物活性分子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号