当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Safety and efficacy of Wharton's jelly‐derived mesenchymal stem cells with teriparatide for osteoporotic vertebral fractures: A phase I/IIa study
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-12-16 , DOI: 10.1002/sctm.20-0308 JeongHyun Shim 1 , Kyoung-Tae Kim 2, 3 , Kwang Gi Kim 4, 5 , Un-Yong Choi 6 , Jae Won Kyung 6 , Seil Sohn 6 , Sang Heon Lim 4 , Hyemin Choi 6 , Tae-Keun Ahn 7 , Hye Jeong Choi 8 , Dong-Eun Shin 7 , Inbo Han 6
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-12-16 , DOI: 10.1002/sctm.20-0308 JeongHyun Shim 1 , Kyoung-Tae Kim 2, 3 , Kwang Gi Kim 4, 5 , Un-Yong Choi 6 , Jae Won Kyung 6 , Seil Sohn 6 , Sang Heon Lim 4 , Hyemin Choi 6 , Tae-Keun Ahn 7 , Hye Jeong Choi 8 , Dong-Eun Shin 7 , Inbo Han 6
Affiliation
|
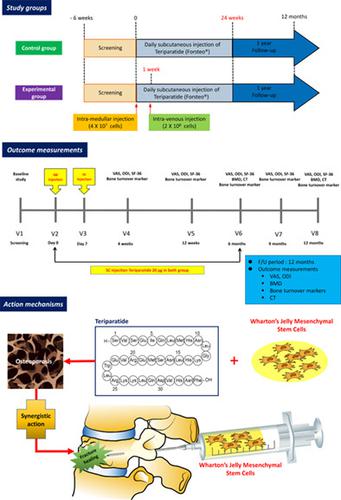
Osteoporotic vertebral compression fractures (OVCFs) are serious health problems. We conducted a randomized, open‐label, phase I/IIa study to determine the feasibility, safety, and effectiveness of Wharton's jelly‐derived mesenchymal stem cells (WJ‐MSCs) and teriparatide (parathyroid hormone 1‐34) in OVCFs. Twenty subjects with recent OVCFs were randomized to teriparatide (20 μg/day, daily subcutaneous injection for 6 months) treatment alone or combined treatment of WJ‐MSCs (intramedullary [4 × 107 cells] injection and intravenous [2 × 108 cells] injection after 1 week) and teriparatide (20 μg/day, daily subcutaneous injection for 6 months). Fourteen subjects (teriparatide alone, n = 7; combined treatment, n = 7) completed follow‐up assessment (visual analog scale [VAS], Oswestry Disability Index [ODI], Short Form‐36 [SF‐36], bone mineral density [BMD], bone turnover measured by osteocalcin and C‐terminal telopeptide of type 1 collagen, dual‐energy x‐ray absorptiometry [DXA], computed tomography [CT]). Our results show that (a) combined treatment with WJ‐MSCs and teriparatide is feasible and tolerable for the patients with OVCFs; (b) the mean VAS, ODI, and SF‐36 scores significantly improved in the combined treatment group; (c) the level of bone turnover markers were not significantly different between the two groups; (d) BMD T‐scores of spine and hip by DXA increased in both control and experimental groups without a statistical difference; and (e) baseline spine CT images and follow‐up CT images at 6 and 12 months showed better microarchitecture in the combined treatment group. Our results indicate that combined treatment of WJ‐MSCs and teriparatide is feasible and tolerable and has a clinical benefit for fracture healing by promoting bone architecture. Clinical trial registration: https://nedrug.mfds.go.kr/, MFDS: 201600282‐30937.
中文翻译:

沃顿胶源性间充质干细胞联合特立帕肽治疗骨质疏松性椎体骨折的安全性和有效性:I/IIa 期研究
骨质疏松性椎体压缩性骨折(OVCF)是严重的健康问题。我们进行了一项随机、开放标签的 I/IIa 期研究,以确定沃顿胶源性间充质干细胞 (WJ-MSC) 和特立帕肽(甲状旁腺激素 1-34)在 OVCF 中的可行性、安全性和有效性。 20 名近期患有 OVCF 的受试者随机接受特立帕肽(20 μg/天,每天皮下注射,持续 6 个月)单独治疗或 WJ-MSC 联合治疗(髓内 [4 × 10 7 个细胞] 注射和静脉注射 [2 × 10 8 个细胞] 1周后注射)和特立帕肽(20μg/天,每天皮下注射,持续6个月)。 14 名受试者(单用特立帕肽,n = 7;联合治疗,n = 7)完成了随访评估(视觉模拟量表 [VAS]、Oswestry 残疾指数 [ODI]、简表‐36 [SF‐36]、骨矿物质密度[BMD]、通过骨钙素和 1 型胶原 C 末端端肽测量的骨转换、双能 X 射线吸收测定法 [DXA]、计算机断层扫描 [CT])。我们的结果表明,(a) WJ-MSC 和特立帕肽联合治疗对于 OVCF 患者是可行且耐受的; (b) 联合治疗组的平均 VAS、ODI 和 SF-36 评分显着改善; (c)两组之间骨转换标志物的水平没有显着差异; (d) 对照组和实验组的 DXA 脊柱和髋部 BMD T 评分均有所增加,但无统计学差异; (e) 基线脊柱 CT 图像以及 6 个月和 12 个月的随访 CT 图像显示联合治疗组有更好的微结构。 我们的结果表明,WJ-MSC 和特立帕肽联合治疗是可行且耐受的,并且通过促进骨结构对骨折愈合具有临床益处。临床试验注册:https://nedrug.mfds.go.kr/,MFDS:201600282‐30937。
更新日期:2020-12-16
中文翻译:

沃顿胶源性间充质干细胞联合特立帕肽治疗骨质疏松性椎体骨折的安全性和有效性:I/IIa 期研究
骨质疏松性椎体压缩性骨折(OVCF)是严重的健康问题。我们进行了一项随机、开放标签的 I/IIa 期研究,以确定沃顿胶源性间充质干细胞 (WJ-MSC) 和特立帕肽(甲状旁腺激素 1-34)在 OVCF 中的可行性、安全性和有效性。 20 名近期患有 OVCF 的受试者随机接受特立帕肽(20 μg/天,每天皮下注射,持续 6 个月)单独治疗或 WJ-MSC 联合治疗(髓内 [4 × 10 7 个细胞] 注射和静脉注射 [2 × 10 8 个细胞] 1周后注射)和特立帕肽(20μg/天,每天皮下注射,持续6个月)。 14 名受试者(单用特立帕肽,n = 7;联合治疗,n = 7)完成了随访评估(视觉模拟量表 [VAS]、Oswestry 残疾指数 [ODI]、简表‐36 [SF‐36]、骨矿物质密度[BMD]、通过骨钙素和 1 型胶原 C 末端端肽测量的骨转换、双能 X 射线吸收测定法 [DXA]、计算机断层扫描 [CT])。我们的结果表明,(a) WJ-MSC 和特立帕肽联合治疗对于 OVCF 患者是可行且耐受的; (b) 联合治疗组的平均 VAS、ODI 和 SF-36 评分显着改善; (c)两组之间骨转换标志物的水平没有显着差异; (d) 对照组和实验组的 DXA 脊柱和髋部 BMD T 评分均有所增加,但无统计学差异; (e) 基线脊柱 CT 图像以及 6 个月和 12 个月的随访 CT 图像显示联合治疗组有更好的微结构。 我们的结果表明,WJ-MSC 和特立帕肽联合治疗是可行且耐受的,并且通过促进骨结构对骨折愈合具有临床益处。临床试验注册:https://nedrug.mfds.go.kr/,MFDS:201600282‐30937。










































 京公网安备 11010802027423号
京公网安备 11010802027423号