当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Convergent Total Synthesis of Yaku'amide A
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-12-17 , DOI: 10.1002/anie.202014238 Yu Cai 1 , Zhiwei Ma 1 , Jintao Jiang 1 , Concordia C L Lo 1 , Shi Luo 1 , Ankur Jalan 1 , Joseph M Cardon 1 , Alexander Ramos 1 , Diego A Moyá 1 , Daniel Joaquin 1 , Steven L Castle 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-12-17 , DOI: 10.1002/anie.202014238 Yu Cai 1 , Zhiwei Ma 1 , Jintao Jiang 1 , Concordia C L Lo 1 , Shi Luo 1 , Ankur Jalan 1 , Joseph M Cardon 1 , Alexander Ramos 1 , Diego A Moyá 1 , Daniel Joaquin 1 , Steven L Castle 1
Affiliation
|
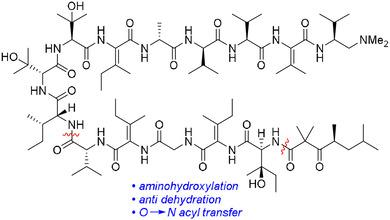
Total synthesis of the anticancer peptide natural product yaku'amide A is reported. Its β‐tert‐hydroxy amino acids were prepared by regioselective aminohydroxylation involving a chiral mesyloxycarbamate reagent. Stereospecific construction of the E‐ and Z‐ΔIle residues was accomplished through a one‐pot reaction featuring anti dehydration, azide reduction, and O→N acyl transfer. Alkene isomerization was negligible during this process. These methods enabled a highly convergent and efficient synthetic route to the natural product.
中文翻译:

Yaku'amide A的聚合全合成
报道了抗癌肽天然产物yaku'amide A的全合成。它的β-叔羟基氨基酸是通过区域选择性氨基羟基化反应制备的,涉及手性甲磺氧基氨基甲酸酯试剂。E-和Z -ΔIle残基的立体特异性构建是通过具有抗脱水、叠氮化物还原和O→N酰基转移的单锅反应完成的。在此过程中烯烃异构化可忽略不计。这些方法为天然产物提供了一种高度收敛和有效的合成路线。
更新日期:2021-02-22
中文翻译:

Yaku'amide A的聚合全合成
报道了抗癌肽天然产物yaku'amide A的全合成。它的β-叔羟基氨基酸是通过区域选择性氨基羟基化反应制备的,涉及手性甲磺氧基氨基甲酸酯试剂。E-和Z -ΔIle残基的立体特异性构建是通过具有抗脱水、叠氮化物还原和O→N酰基转移的单锅反应完成的。在此过程中烯烃异构化可忽略不计。这些方法为天然产物提供了一种高度收敛和有效的合成路线。









































 京公网安备 11010802027423号
京公网安备 11010802027423号