当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Arsenite oxyanions affect CeO2 nanoparticle dissolution and colloidal stability
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-11-25 , DOI: 10.1039/d0en00970a Chelsea W. Neil 1, 2, 3, 4, 5 , Xuanhao Wu 1, 2, 3, 4, 5 , Doyoon Kim 1, 2, 3, 4, 5 , Haesung Jung 1, 2, 3, 4, 5 , Yanzhe Zhu 1, 2, 3, 4, 5 , Jessica R. Ray 1, 2, 3, 4, 5 , Young-Shin Jun 1, 2, 3, 4, 5
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-11-25 , DOI: 10.1039/d0en00970a Chelsea W. Neil 1, 2, 3, 4, 5 , Xuanhao Wu 1, 2, 3, 4, 5 , Doyoon Kim 1, 2, 3, 4, 5 , Haesung Jung 1, 2, 3, 4, 5 , Yanzhe Zhu 1, 2, 3, 4, 5 , Jessica R. Ray 1, 2, 3, 4, 5 , Young-Shin Jun 1, 2, 3, 4, 5
Affiliation
|
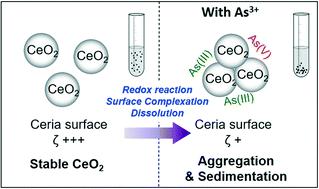
While highly reactive cerium oxide nanoparticles (CeO2 NPs) are widely used in industry, their transport in aquatic systems is not well understood. To fill this knowledge gap, the interactions of CeO2 NPs with arsenite (As3+), a toxic metalloid and potential co-present contaminant, were investigated with respect to CeO2 NP colloidal stability, dissolution, and surface redox reactions. Arsenite showed distinctive effects at different concentrations, with a high As3+ concentration (10−4 M) inducing 90% of CeO2 NPs to settle from solution after 8 hours, while lower As3+ concentrations (10−5 or 10−6 M) led to only 20% of CeO2 NPs settling. The dissolution of NPs was most significant in the 10−5 M As3+ system owing to a lesser extent of aggregation, exposing more CeO2 surface for dissolution. In the three As3+ concentration systems, >97% of aqueous arsenic remained as As3+ over 6 hours. On the NP surface, adsorbed AsIII was oxidized to AsV, resulting in 58–70% of the adsorbed arsenic remaining as AsIII. Simultaneously CeIV was reduced to CeIII, increasing CeIII on the CeO2 NP surface from 17% (without arsenite) to 21–25% (with arsenite). Further mechanistic analyses revealed that the adsorption of arsenite was the main contributor to neutralizing the CeO2 NP surface potential, enhancing particle sedimentation. These findings suggest that the fate and transport of CeO2 NPs in our experimental systems are strongly affected by arsenite concentration and its adsorption on NPs. The results also highlight the importance of the interplay between NP aggregation, oxidation, and dissolution in predicting the behaviors of CeO2 NPs and associated toxic elements in aquatic systems.
中文翻译:

砷氧根阴离子影响CeO2纳米颗粒的溶解和胶体稳定性
尽管高反应性氧化铈纳米颗粒(CeO 2 NPs)在工业中得到了广泛使用,但人们对其在水生系统中的运输却知之甚少。为了填补这一知识空白,针对CeO 2 NP的胶体稳定性,溶解性和表面氧化还原反应,研究了CeO 2 NP与亚砷酸盐(As 3+),有毒的准金属和潜在的共存污染物的相互作用。砷在不同浓度下表现出独特的作用,高浓度的As 3+(10 -4 M)会在8小时后诱导90%的CeO 2 NP从溶液中沉降,而较低的As 3+浓度(10 -5或10-6 M)仅导致20%的CeO 2 NP沉降。由于聚集程度较小,NPs的溶解在10 -5 M As 3+系统中最显着,暴露出更多的CeO 2表面用于溶解。在三个As 3+浓度系统中,在6小时内> 97%的砷3+水溶液残留。在NP表面,吸附的As III被氧化为As V,导致58-70%的砷吸附残留为As III。同时将Ce IV还原为Ce III,在CeO 2上增加Ce IIINP表面从17%(无砷)到21–25%(有砷)。进一步的机理分析表明,砷的吸附是中和CeO 2 NP表面电位,促进颗粒沉降的主要因素。这些发现表明,我们的实验系统中CeO 2 NP的命运和运输受到亚砷酸盐浓度及其在NP上的吸附的强烈影响。结果还突出了NP聚集,氧化和溶解之间相互作用的重要性,以预测CeO 2 NPs及其相关毒性元素在水生系统中的行为。
更新日期:2020-12-17
中文翻译:

砷氧根阴离子影响CeO2纳米颗粒的溶解和胶体稳定性
尽管高反应性氧化铈纳米颗粒(CeO 2 NPs)在工业中得到了广泛使用,但人们对其在水生系统中的运输却知之甚少。为了填补这一知识空白,针对CeO 2 NP的胶体稳定性,溶解性和表面氧化还原反应,研究了CeO 2 NP与亚砷酸盐(As 3+),有毒的准金属和潜在的共存污染物的相互作用。砷在不同浓度下表现出独特的作用,高浓度的As 3+(10 -4 M)会在8小时后诱导90%的CeO 2 NP从溶液中沉降,而较低的As 3+浓度(10 -5或10-6 M)仅导致20%的CeO 2 NP沉降。由于聚集程度较小,NPs的溶解在10 -5 M As 3+系统中最显着,暴露出更多的CeO 2表面用于溶解。在三个As 3+浓度系统中,在6小时内> 97%的砷3+水溶液残留。在NP表面,吸附的As III被氧化为As V,导致58-70%的砷吸附残留为As III。同时将Ce IV还原为Ce III,在CeO 2上增加Ce IIINP表面从17%(无砷)到21–25%(有砷)。进一步的机理分析表明,砷的吸附是中和CeO 2 NP表面电位,促进颗粒沉降的主要因素。这些发现表明,我们的实验系统中CeO 2 NP的命运和运输受到亚砷酸盐浓度及其在NP上的吸附的强烈影响。结果还突出了NP聚集,氧化和溶解之间相互作用的重要性,以预测CeO 2 NPs及其相关毒性元素在水生系统中的行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号