Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Traceless Redox-Annulations of Alicyclic Amines
SynOpen Pub Date : 2020-12-16 , DOI: 10.1055/s-0040-1706004 Dillon R L Rickertsen 1 , Longle Ma 2 , Anirudra Paul 1 , Khalil A Abboud 3 , Daniel Seidel 1
中文翻译:

脂环胺的无痕氧化还原环化
更新日期:2020-12-17
SynOpen Pub Date : 2020-12-16 , DOI: 10.1055/s-0040-1706004 Dillon R L Rickertsen 1 , Longle Ma 2 , Anirudra Paul 1 , Khalil A Abboud 3 , Daniel Seidel 1
Affiliation
|
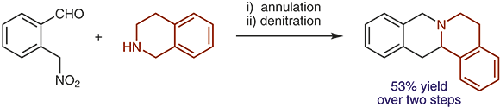
Amines such as 1,2,3,4-tetrahydroisoquinoline undergo redox-neutral annulations with ortho-(nitromethyl)benzaldehyde. Benzoic acid acts as a promoter in these reactions, which involve concurrent amine α-C–H bond and N–H bond functionalization. Subsequent removal of the nitro group provides access to tetrahydroprotoberberines not accessible via typical redox-annulations. Also reported are decarboxylative annulations of ortho-(nitromethyl)benzaldehyde with proline and pipecolic acid.
中文翻译:

脂环胺的无痕氧化还原环化
1,2,3,4-四氢异喹啉等胺与邻-(硝基甲基)苯甲醛发生氧化还原中性环化。苯甲酸在这些反应中充当促进剂,涉及同时的胺 α-C-H 键和 N-H 键官能化。随后硝基的去除提供了通过典型的氧化还原环化无法获得的四氢原小檗碱。还报道了邻-(硝基甲基)苯甲醛与脯氨酸和哌可酸的脱羧环化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号