当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Triazolization of Enolizable Ketones with Primary Amines: A General Strategy toward Multifunctional 1,2,3‐Triazoles
The Chemical Record ( IF 7.0 ) Pub Date : 2020-12-17 , DOI: 10.1002/tcr.202000151 Rashmi Prakash 1 , Tomas Opsomer 1 , Wim Dehaen 1
The Chemical Record ( IF 7.0 ) Pub Date : 2020-12-17 , DOI: 10.1002/tcr.202000151 Rashmi Prakash 1 , Tomas Opsomer 1 , Wim Dehaen 1
Affiliation
|
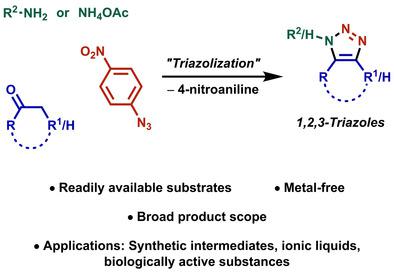
The development of metal‐free syntheses toward 1,2,3‐triazoles has been a burgeoning research area throughout the past decade. Despite the numerous advances, the scarceness of methods for the preparation of 1,5‐disubstituted 1,2,3‐triazoles from readily available substrates remained a challenge that was addressed by our group in 2016. A metal‐free three‐component reaction, which we have dubbed the triazolization reaction, was established for the rapid synthesis of 1,5‐disubstituted, fully functionalized and NH‐1,2,3‐triazoles. This novel approach stands out because it utilizes widely available starting materials, namely primary amines and enolizable ketones. Furthermore, the broad substrate scope is a major advantage, and was further expanded by the number of modified protocols that have been reported. Triazolization products have successfully found utility as intermediates in various synthetic transformations, and were the subject of a few interesting biological activity studies.
中文翻译:

烯醇化酮与伯胺的三唑化:多功能 1,2,3-三唑的通用策略
在过去的十年中,1,2,3-三唑的无金属合成的发展一直是一个新兴的研究领域。尽管取得了许多进展,但从容易获得的底物中制备 1,5-二取代 1,2,3-三唑的方法的稀缺仍然是我们小组在 2016 年解决的一个挑战。一种无金属的三组分反应,我们将其称为三唑化反应,旨在快速合成 1,5-二取代、全功能化和NH -1,2,3-三唑。这种新颖的方法之所以引人注目,是因为它利用了广泛可用的起始材料,即伯胺和烯醇化酮。此外,广泛的底物范围是一个主要优势,并且通过已报告的修改方案的数量进一步扩大。三唑化产物已成功地在各种合成转化中找到了作为中间体的用途,并且是一些有趣的生物活性研究的主题。
更新日期:2021-02-18
中文翻译:

烯醇化酮与伯胺的三唑化:多功能 1,2,3-三唑的通用策略
在过去的十年中,1,2,3-三唑的无金属合成的发展一直是一个新兴的研究领域。尽管取得了许多进展,但从容易获得的底物中制备 1,5-二取代 1,2,3-三唑的方法的稀缺仍然是我们小组在 2016 年解决的一个挑战。一种无金属的三组分反应,我们将其称为三唑化反应,旨在快速合成 1,5-二取代、全功能化和NH -1,2,3-三唑。这种新颖的方法之所以引人注目,是因为它利用了广泛可用的起始材料,即伯胺和烯醇化酮。此外,广泛的底物范围是一个主要优势,并且通过已报告的修改方案的数量进一步扩大。三唑化产物已成功地在各种合成转化中找到了作为中间体的用途,并且是一些有趣的生物活性研究的主题。











































 京公网安备 11010802027423号
京公网安备 11010802027423号