当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Complex crystal structure determination and anti‐non‐small‐cell lung cancer activity of the Hsp90N inhibitor Debio0932
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-12-17 , DOI: 10.1107/s2059798320014990 Wei Qin 1 , Feng Yu 2 , Huan Zhou 2 , Ping Li 1 , Fang Zhou 1 , Hui Jin Li 1 , Chun Xia He 1 , Lu Xing 1 , Xin Zhou 1 , Dong Zhao 1 , Peng Quan Li 1 , Xi Jin 1 , Qi Sheng Wang 2 , Jian Hua He 2 , Hui Ling Cao 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-12-17 , DOI: 10.1107/s2059798320014990 Wei Qin 1 , Feng Yu 2 , Huan Zhou 2 , Ping Li 1 , Fang Zhou 1 , Hui Jin Li 1 , Chun Xia He 1 , Lu Xing 1 , Xin Zhou 1 , Dong Zhao 1 , Peng Quan Li 1 , Xi Jin 1 , Qi Sheng Wang 2 , Jian Hua He 2 , Hui Ling Cao 1
Affiliation
|
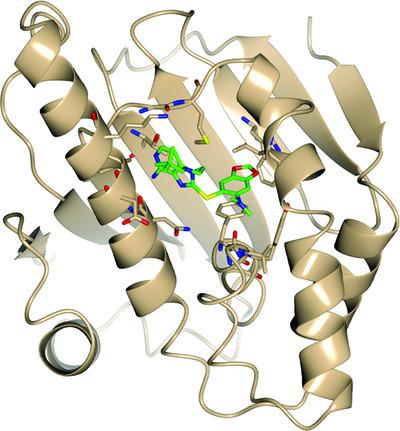
Debio0932 is a promising lead compound in phase I clinical trials targeting the N‐terminal ATP‐binding pocket of the molecular chaperone heat‐shock protein 90 (Hsp90N). The absence of a crystal structure of the Hsp90N–Debio0932 complex, however, has impeded further structural optimization of Debio0932 and understanding of the molecular‐interaction mechanism. Here, a high‐resolution crystal structure of the Hsp90N–Debio0932 complex was successfully determined (resolution limit 2.20 Å; PDB entry 6lr9) by X‐ray diffraction and the molecular‐interaction mechanism was analysed in detail, which suggested that Debio0932 suppresses cancer cells by accommodating itself in the ATP‐binding pocket of Hsp90N, disabling its molecular‐chaperone capability. The results of a thermal shift assay (ΔTm = 8.83 ± 0.90°C) and isothermal titration calorimetry (Kd = 15.50 ± 1.30 nM) indicated strong binding and favourable thermodynamic changes in the binding of Hsp90N and Debio0932. Based on the crystal structure of the complex and on molecular‐interaction analysis, 30 new Debio0932 derivatives were designed and nine new derivatives exhibited increased binding to Hsp90N, as determined by molecular‐docking evaluation. Additionally, Debio0932 suppressed cell proliferation (IC50 values of 3.26 ± 2.82 µM for A549, 20.33 ± 5.39 µM for H1299 and 3.16 ± 1.04 µM for H1975), induced cell‐cycle arrest and promoted apoptosis in three non‐small‐cell lung cancer (NSCLC) cell lines. These results provide novel perspectives and guidance for the development of new anti‐NSCLC drugs based on the lead compound Debio0932.
中文翻译:

Hsp90N抑制剂Debio0932的复杂晶体结构测定和抗非小细胞肺癌活性
在针对分子伴侣热休克蛋白 90 (Hsp90 N )的 N 端 ATP 结合口袋的 I 期临床试验中,Debio0932 是一种很有前景的先导化合物。然而,Hsp90 N- Debio0932 复合物的晶体结构的缺失阻碍了 Debio0932 的进一步结构优化和对分子相互作用机制的理解。在这里,通过 X 射线衍射成功确定了 Hsp90 N- Debio0932 复合物的高分辨率晶体结构(分辨率极限 2.20 Å;PDB 条目 6lr9),并详细分析了分子相互作用机制,这表明 Debio0932 抑制癌症通过将自身容纳在 Hsp90 N的 ATP 结合口袋中,禁用其分子伴侣能力。热位移测定 (Δ T m = 8.83 ± 0.90°C) 和等温滴定量热法 ( K d = 15.50 ± 1.30 n M ) 的结果表明 Hsp90 N和 Debio0932的结合具有强结合和有利的热力学变化。根据复合物的晶体结构和分子相互作用分析,设计了 30 种新的 Debio0932 衍生物,并通过分子对接评估确定了9 种新衍生物与 Hsp90 N 的结合增加。此外,Debio0932 抑制细胞增殖(A549 的IC 50值为 3.26 ± 2.82 µ M,20.33 ± 5.39 µ M对于 H1299 和 3.16 ± 1.04 µM,对于 H1975),在三种非小细胞肺癌 (NSCLC) 细胞系中诱导细胞周期停滞并促进细胞凋亡。这些结果为开发基于先导化合物 Debio0932 的新型抗 NSCLC 药物提供了新的视角和指导。
更新日期:2021-01-06
中文翻译:

Hsp90N抑制剂Debio0932的复杂晶体结构测定和抗非小细胞肺癌活性
在针对分子伴侣热休克蛋白 90 (Hsp90 N )的 N 端 ATP 结合口袋的 I 期临床试验中,Debio0932 是一种很有前景的先导化合物。然而,Hsp90 N- Debio0932 复合物的晶体结构的缺失阻碍了 Debio0932 的进一步结构优化和对分子相互作用机制的理解。在这里,通过 X 射线衍射成功确定了 Hsp90 N- Debio0932 复合物的高分辨率晶体结构(分辨率极限 2.20 Å;PDB 条目 6lr9),并详细分析了分子相互作用机制,这表明 Debio0932 抑制癌症通过将自身容纳在 Hsp90 N的 ATP 结合口袋中,禁用其分子伴侣能力。热位移测定 (Δ T m = 8.83 ± 0.90°C) 和等温滴定量热法 ( K d = 15.50 ± 1.30 n M ) 的结果表明 Hsp90 N和 Debio0932的结合具有强结合和有利的热力学变化。根据复合物的晶体结构和分子相互作用分析,设计了 30 种新的 Debio0932 衍生物,并通过分子对接评估确定了9 种新衍生物与 Hsp90 N 的结合增加。此外,Debio0932 抑制细胞增殖(A549 的IC 50值为 3.26 ± 2.82 µ M,20.33 ± 5.39 µ M对于 H1299 和 3.16 ± 1.04 µM,对于 H1975),在三种非小细胞肺癌 (NSCLC) 细胞系中诱导细胞周期停滞并促进细胞凋亡。这些结果为开发基于先导化合物 Debio0932 的新型抗 NSCLC 药物提供了新的视角和指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号