当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
d‐Amino acid substitutions and dimerization increase the biological activity and stability of an IL‐15 antagonist peptide
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2020-12-16 , DOI: 10.1002/psc.3293 Yunier Rodríguez-Álvarez 1 , Ania Cabrales-Rico 2 , David Diago-Abreu 2 , Elianys Correa-Arguelles 1 , Osvaldo Reyes-Acosta 2 , Pedro Puente-Pérez 3 , Dagmara Pichardo-Díaz 3 , Dioslaida Urquiza-Noa 3 , Amalia Hernández-Santana 3 , Hilda E Garay-Pérez 2
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2020-12-16 , DOI: 10.1002/psc.3293 Yunier Rodríguez-Álvarez 1 , Ania Cabrales-Rico 2 , David Diago-Abreu 2 , Elianys Correa-Arguelles 1 , Osvaldo Reyes-Acosta 2 , Pedro Puente-Pérez 3 , Dagmara Pichardo-Díaz 3 , Dioslaida Urquiza-Noa 3 , Amalia Hernández-Santana 3 , Hilda E Garay-Pérez 2
Affiliation
|
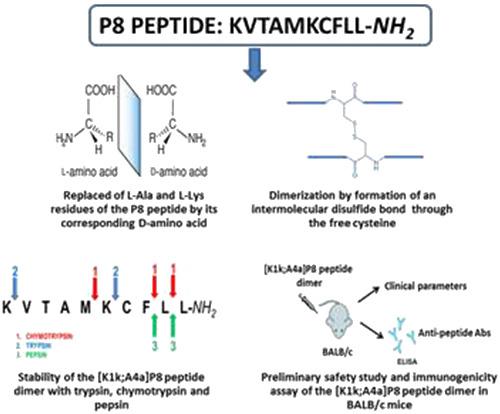
Interleukin (IL)‐15 plays an important role in several inflammatory diseases. We have previously identified an IL‐15 antagonist called P8 peptide, which binds specifically to IL‐15 receptor alpha subunit. However, the P8 peptide rapidly degraded by proteases, limiting its therapeutic application. Thus, we replaced each P8 peptide l‐amino acid by its corresponding d‐isomers. First, we determined the biological activity of the resulting peptides in a proliferation assay by using CTLL‐2 cells. The substitution of l‐Ala by d‐Ala ([A4a]P8 peptide) increased the inhibitory effect of the P8 peptide in CTLL‐2 cells in five‐fold. In addition to that, the [A4a]P8 peptide dimer showed the most inhibitory effect. To protect the [A4a]P8 peptide and its dimer against exopeptidase activity, we acetylated the N‐terminal of these peptides. At least a three‐fold reduction in antagonist activity of acetylated peptides was exhibited. However, the substitution of the N‐terminal l‐Lys residue of [A4a]P8 peptide and its dimer by d‐Lys ([K1k;A4a]P8 peptide) did not affect the antagonist effect of the aforementioned peptides. The [K1k;A4a]P8 peptide dimer was stable to the degradation of trypsin, chymotrypsin, and pepsin up until 48 min. Also, the safety and immunogenicity studies in healthy BALB/c mice demonstrated that the administration of this peptide did not affect the clinical parameters of the animals nor generated antipeptide antibodies. Our findings reveal that two distinct d‐amino acid substitutions and dimerization increase the biological activity and stability of P8 peptide. The resulting peptide constitutes a novel IL‐15 antagonist with potential applicability in inflammatory diseases.
中文翻译:

d-氨基酸取代和二聚化增加了 IL-15 拮抗剂肽的生物活性和稳定性
白细胞介素 (IL)-15 在多种炎症性疾病中发挥重要作用。我们之前已经确定了一种称为 P8 肽的 IL-15 拮抗剂,它与 IL-15 受体 α 亚基特异性结合。然而,P8 肽被蛋白酶迅速降解,限制了其治疗应用。因此,我们用相应的d-异构体替换了每个 P8 肽的l-氨基酸。首先,我们使用 CTLL-2 细胞在增殖试验中测定了所得肽的生物活性。l- Ala 被d取代‐Ala([A4a]P8 肽)将 P8 肽对 CTLL-2 细胞的抑制作用提高了 5 倍。除此之外,[A4a]P8 肽二聚体显示出最大的抑制作用。为了保护 [A4a]P8 肽及其二聚体免受外肽酶活性的影响,我们将这些肽的 N 端乙酰化。乙酰化肽的拮抗剂活性至少降低了三倍。然而,[A4a]P8 肽及其二聚体的 N 端l - Lys 残基被d取代‐Lys ([K1k;A4a]P8 肽) 不影响上述肽的拮抗作用。[K1k;A4a]P8 肽二聚体对胰蛋白酶、胰凝乳蛋白酶和胃蛋白酶的降解保持稳定,直至 48 分钟。此外,在健康 BALB/c 小鼠中的安全性和免疫原性研究表明,这种肽的给药不会影响动物的临床参数,也不会产生抗肽抗体。我们的研究结果表明,两个不同的d-氨基酸取代和二聚化增加了 P8 肽的生物活性和稳定性。由此产生的肽构成了一种新型的 IL-15 拮抗剂,在炎症性疾病中具有潜在的适用性。
更新日期:2021-01-28
中文翻译:

d-氨基酸取代和二聚化增加了 IL-15 拮抗剂肽的生物活性和稳定性
白细胞介素 (IL)-15 在多种炎症性疾病中发挥重要作用。我们之前已经确定了一种称为 P8 肽的 IL-15 拮抗剂,它与 IL-15 受体 α 亚基特异性结合。然而,P8 肽被蛋白酶迅速降解,限制了其治疗应用。因此,我们用相应的d-异构体替换了每个 P8 肽的l-氨基酸。首先,我们使用 CTLL-2 细胞在增殖试验中测定了所得肽的生物活性。l- Ala 被d取代‐Ala([A4a]P8 肽)将 P8 肽对 CTLL-2 细胞的抑制作用提高了 5 倍。除此之外,[A4a]P8 肽二聚体显示出最大的抑制作用。为了保护 [A4a]P8 肽及其二聚体免受外肽酶活性的影响,我们将这些肽的 N 端乙酰化。乙酰化肽的拮抗剂活性至少降低了三倍。然而,[A4a]P8 肽及其二聚体的 N 端l - Lys 残基被d取代‐Lys ([K1k;A4a]P8 肽) 不影响上述肽的拮抗作用。[K1k;A4a]P8 肽二聚体对胰蛋白酶、胰凝乳蛋白酶和胃蛋白酶的降解保持稳定,直至 48 分钟。此外,在健康 BALB/c 小鼠中的安全性和免疫原性研究表明,这种肽的给药不会影响动物的临床参数,也不会产生抗肽抗体。我们的研究结果表明,两个不同的d-氨基酸取代和二聚化增加了 P8 肽的生物活性和稳定性。由此产生的肽构成了一种新型的 IL-15 拮抗剂,在炎症性疾病中具有潜在的适用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号