当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering soluble artificial epidermal growth factor receptor mimics capable of spontaneous in vitro dimerization
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-12-17 , DOI: 10.1002/bit.27659 Allison Sunderhaus 1 , Ramsha Imran 1 , Amanda Goudelock 1 , Manon Nassar 2 , Kendall Cooper 2 , Dustin Patterson 2 , May H Abdel Aziz 1
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2020-12-17 , DOI: 10.1002/bit.27659 Allison Sunderhaus 1 , Ramsha Imran 1 , Amanda Goudelock 1 , Manon Nassar 2 , Kendall Cooper 2 , Dustin Patterson 2 , May H Abdel Aziz 1
Affiliation
|
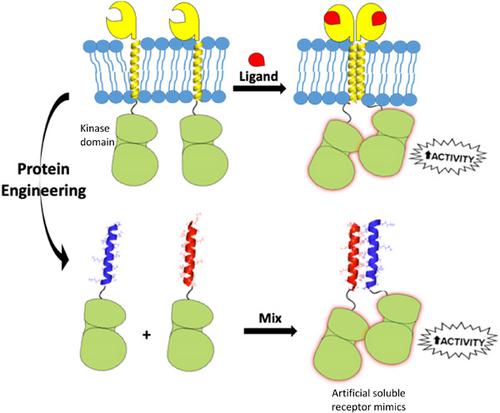
Epidermal growth factor receptor (EGFR) is a clinically validated target for a multitude of human cancers. The receptor is activated upon ligand binding through a critical dimerization step. Dimerization can be replicated in vitro by locally concentrating the receptor kinase domains on the surface of lipid‐based vesicles. In this study we investigated the use of coiled coils to induce spontaneous receptor kinase domain dimerization in vitro to form non‐membrane‐bound artificial receptor mimics in solution. Two engineered forms of EGFR kinase domain fused to coiled coil complementary peptides were designed to self‐associate upon mixing. Two fusion protein species (P3‐EGFR and P4‐EGFR) independently showed the same activity and polymerization profile known to exist with EGFR kinase domains. Upon mixing the two species, coiled coil heterodimers were formed that induced EGFR association to form dimers of the kinase domains. This was accompanied by 11.5‐fold increase in the phosphorylation rate indicative of kinase domain activation equivalent to the levels achieved using vesicle localization and mimicking in vivo ligand‐induced activation. This study presents a soluble tyrosine kinase receptor mimic capable of spontaneous in vitro activation that can facilitate functional and drug discovery studies for this clinically important receptor class.
中文翻译:

工程化可溶性人工表皮生长因子受体模拟物,能够在体外自发二聚化
表皮生长因子受体 (EGFR) 是多种人类癌症的临床验证靶标。受体通过关键的二聚化步骤在配体结合后被激活。通过将受体激酶结构域局部集中在基于脂质的囊泡表面,可以在体外复制二聚化。在这项研究中,我们研究了使用盘绕线圈在体外诱导自发受体激酶结构域二聚化,从而在溶液中形成非膜结合的人工受体模拟物。与卷曲螺旋互补肽融合的两种工程形式的 EGFR 激酶结构域被设计为在混合时自缔合。两种融合蛋白种类(P3-EGFR 和 P4-EGFR)独立地显示出与 EGFR 激酶结构域相同的活性和聚合特征。将这两种物种混合后,形成卷曲螺旋异二聚体,诱导EGFR结合形成激酶结构域的二聚体。这伴随着磷酸化率增加了 11.5 倍,这表明激酶域激活相当于使用囊泡定位和模拟体内配体诱导的激活所达到的水平。本研究提出了一种可溶性酪氨酸激酶受体模拟物,能够在体外自发激活,从而促进这一临床重要受体类别的功能和药物发现研究。
更新日期:2020-12-17
中文翻译:

工程化可溶性人工表皮生长因子受体模拟物,能够在体外自发二聚化
表皮生长因子受体 (EGFR) 是多种人类癌症的临床验证靶标。受体通过关键的二聚化步骤在配体结合后被激活。通过将受体激酶结构域局部集中在基于脂质的囊泡表面,可以在体外复制二聚化。在这项研究中,我们研究了使用盘绕线圈在体外诱导自发受体激酶结构域二聚化,从而在溶液中形成非膜结合的人工受体模拟物。与卷曲螺旋互补肽融合的两种工程形式的 EGFR 激酶结构域被设计为在混合时自缔合。两种融合蛋白种类(P3-EGFR 和 P4-EGFR)独立地显示出与 EGFR 激酶结构域相同的活性和聚合特征。将这两种物种混合后,形成卷曲螺旋异二聚体,诱导EGFR结合形成激酶结构域的二聚体。这伴随着磷酸化率增加了 11.5 倍,这表明激酶域激活相当于使用囊泡定位和模拟体内配体诱导的激活所达到的水平。本研究提出了一种可溶性酪氨酸激酶受体模拟物,能够在体外自发激活,从而促进这一临床重要受体类别的功能和药物发现研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号