Physica D: Nonlinear Phenomena ( IF 2.7 ) Pub Date : 2020-12-17 , DOI: 10.1016/j.physd.2020.132813 Stavros C. Farantos
|
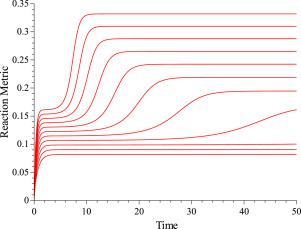
Classical thermodynamics have recently been formulated as Hamiltonian systems elevating the theory of heat to the same level of description as analytical mechanics. Particularly, mechanical and thermodynamical systems can merge in a single dynamical theory to describe equilibrium and non-equilibrium processes by constructing homogeneous of first degree in momenta Hamiltonian functions on the extended thermodynamic phase space and in a variety of representations. By employing geometric Hamiltonian theory we show that classical chemical kinetics can also be put in a Hamiltonian framework with Massieu–Gibbs function as the generating Hamiltonian at constant temperature and pressure. A metric in the physical state manifold as well as the entropy production in irreversible chemical reactions are defined. This way we establish a common computational platform for chemical kinetics and chemical dynamics. Numerical results are presented from the study of consecutive first-order elementary reactions and the non-linear kinetic equations of a model for autocatalytic symmetry breaking chiral reactions. For the latter example, we have examined two cases; that of a closed system with fixed initial concentrations and the steady-state case.
中文翻译:

哈密顿经典热力学和化学动力学
近年来,经典的热力学已被表述为哈密顿体系,将热理论提升到与分析力学相同的描述水平。特别是,机械和热力学系统可以通过在扩展的热力学相空间上以各种表示形式构造动量哈密顿函数的一阶均质性,从而在一个单一的动力学理论中合并以描述平衡和非平衡过程。通过采用几何哈密顿理论,我们证明了经典化学动力学也可以置于具有Massieu–Gibbs函数作为在恒定温度和压力下生成哈密顿的哈密顿框架中。定义了物理状态流形中的度量以及不可逆化学反应中的熵产生。这样,我们为化学动力学和化学动力学建立了通用的计算平台。通过对连续一阶基本反应的研究和自催化对称性拆分手性反应模型的非线性动力学方程,给出了数值结果。对于后一个示例,我们研究了两种情况。具有固定初始浓度和稳态情况的密闭系统。











































 京公网安备 11010802027423号
京公网安备 11010802027423号